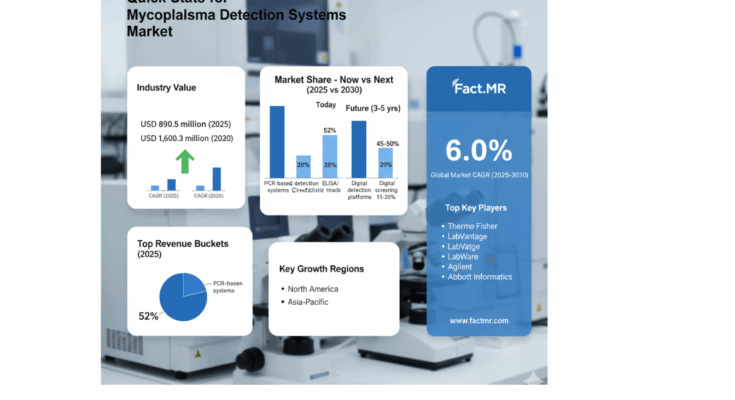
The Mycoplasma Detection Systems Market is poised for transformative growth over the next decade, signaling a compelling opportunity for manufacturers and industry leaders to invest in advanced contamination detection solutions that are reshaping pharmaceutical quality control, biotechnology operations, and research applications worldwide. According to the latest market intelligence, the global market is projected to grow from USD 780 million in 2025 to USD 1.6 billion by 2035, reflecting a robust CAGR of 7.0%.
Mycoplasma Detection Systems Market Accelerating Adoption of Molecular Detection Technologies
The first half of the forecast period (2025–2030) anticipates the market expanding from USD 780 million to USD 1.1 billion, accounting for 41% of the overall decade-long growth. This initial growth phase is predominantly driven by the increasing adoption of PCR-based detection systems, which now represent the leading technology segment with a 52% market share. These systems provide high accuracy and rapid results, critical for pharmaceutical quality control and cell culture contamination prevention. Enhanced molecular detection and automation have become baseline expectations for facilities seeking operational efficiency and regulatory compliance.
Between 2030 and 2035, the market is expected to reach USD 1.6 billion, adding USD 500 million and representing 59% of the total decade growth. During this period, high-throughput detection systems, next-generation PCR technologies, and AI-enabled digital detection platforms will gain widespread adoption. Seamless integration with laboratory information management systems (LIMS) and existing pharmaceutical infrastructure will enable facilities to streamline quality control processes and ensure real-time contamination monitoring, setting new industry standards for operational excellence.
Mycoplasma Detection Systems Market Segmentation Highlights
-
By Technology: PCR-based detection systems dominate the market, followed by ELISA/enzymatic assays (28%) and direct culture methods (20%). Next-generation PCR, qPCR, and AI-enabled detection systems are projected to drive future growth, addressing the demand for multiplexing, real-time analytics, and automated workflow integration.
-
By Application: Cell line testing leads with 48% market share, reflecting widespread adoption in biopharmaceutical production, followed by vaccine and biologics manufacturing (34%) and other applications including veterinary diagnostics, environmental monitoring, and food safety testing (18%).
-
By End User: Pharmaceutical and biotechnology companies command 61% of the market, driven by the need for validated detection methods, regulatory compliance, and operational efficiency. Research institutions and diagnostic laboratories constitute the remaining demand, highlighting opportunities for specialized detection solutions.
-
By Region: North America continues to lead market adoption, with the USA at a 7.5% growth rate due to mature biopharmaceutical infrastructure and regulatory frameworks. Europe demonstrates steady expansion, with Germany, France, and the UK as key markets. East Asia, particularly South Korea and Japan, is experiencing strong growth driven by biotechnology advancement and manufacturing modernization initiatives.
Mycoplasma Detection Systems Market Key Growth Drivers
The Mycoplasma Detection Systems Market is anchored by several key drivers:
-
Biopharmaceutical Production & Cell Therapy Expansion: Increasing cell culture applications necessitate comprehensive contamination screening and validated detection protocols.
-
Regulatory Compliance & Quality Standards: Regulatory mandates from FDA, EMA, and ICH guidelines transform detection systems from optional to essential, creating demand for validated, reliable technologies.
-
Contract Manufacturing & Outsourcing: The rise of CMOs requires multi-client capable systems capable of rapid, high-throughput testing and contamination monitoring.
-
Technological Advancements: AI-powered analytics, automated sample processing, and digital result interpretation improve laboratory efficiency and reduce human error.
Mycoplasma Detection Systems Market Challenges
Despite robust growth, certain restraints persist, including high equipment costs and validation expenses, technical complexity requiring skilled operators, and infrastructure limitations in emerging markets. These factors present opportunities for manufacturers to offer value-added services, training, and scalable deployment options.
Mycoplasma Detection Systems Market Competitive Landscape
The market is highly competitive, with approximately 12–15 credible players dominating global revenue. Leading companies include Lonza, Sartorius, Thermo Fisher Scientific, Charles River Laboratories, Merck Millipore, Agilent Technologies, Qiagen, Takara Bio, PromoCell, and ATCC. Market leadership is maintained through technology innovation, regulatory expertise, and integration into laboratory workflows. Opportunities for margin expansion lie in validation services, regulatory support, and digital integration platforms that enhance throughput and compliance tracking.
Mycoplasma Detection Systems Market Strategic Implications for Industry Leaders
For manufacturers and industry stakeholders, the market underscores the importance of designing solutions for regulatory compliance, scalability, and automation. Key imperatives include:
-
Offering comprehensive detection solutions with preconfigured workflows, validation documentation, and trained technical support.
-
Ensuring digital integration for LIMS connectivity, real-time monitoring, and trend analytics.
-
Developing value-based pricing models with clear service tiers and subscription-based digital analytics offerings.
-
Aligning product innovation with emerging regional markets and regulatory landscapes to maximize adoption and operational impact.
Mycoplasma Detection Systems Market Regional Opportunities
The USA leads in adoption due to established pharmaceutical and biotech hubs. Germany maintains technology leadership through premium precision detection systems, while France and the UK demonstrate steady adoption fueled by quality assurance and regulatory compliance. South Korea and Mexico show high-growth potential due to biotechnology expansion and increasing pharmaceutical infrastructure investments.
Mycoplasma Detection Systems Market Conclusion
The Mycoplasma Detection Systems Market presents an unprecedented opportunity for manufacturers to participate in a rapidly evolving industry driven by technological innovation, regulatory requirements, and biopharmaceutical expansion. By focusing on molecular detection accuracy, high-throughput automation, and integrated digital platforms, stakeholders can capture emerging growth opportunities, enhance laboratory performance, and reinforce quality assurance standards across global pharmaceutical and biotechnology operations.
Browse Full Report : https://www.factmr.com/report/446/mycoplasma-detection-systems-market