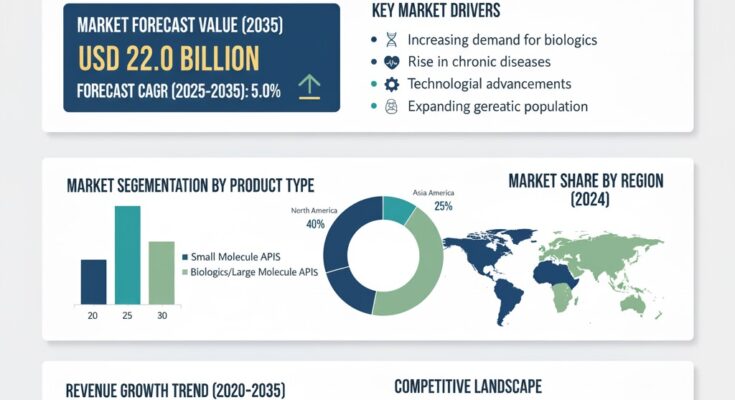
The global sterile active pharmaceutical ingredient market is witnessing steady expansion, fueled by the rising demand for injectable drugs, biologics, and high-purity formulations. According to a recent report by Fact.MR, the market is valued at USD 13.5 billion in 2025 and is projected to reach USD 22.0 billion by 2035, reflecting an absolute increase of USD 8.5 billion over the forecast period. This growth translates into a compound annual growth rate (CAGR) of 5.0% between 2025 and 2035.
As the pharmaceutical sector shifts toward complex biologics and sterile dosage forms, the demand for sterile APIs has surged across global manufacturing facilities. These high-purity ingredients form the backbone of life-saving injectable and ophthalmic drugs, requiring stringent aseptic processing, regulatory compliance, and advanced manufacturing technologies.
Market Drivers: Biopharmaceutical Growth, Quality Regulations, and Technological Advancements
Rising Demand for Injectable Drugs and Biologics
The expanding use of biologics and parenteral drugs for treating chronic diseases such as cancer, diabetes, and autoimmune disorders is a major catalyst for sterile API demand. These formulations require contamination-free, sterile environments to maintain efficacy and safety, driving pharmaceutical manufacturers to enhance sterile production capacities globally.
Stringent Regulatory Frameworks and GMP Compliance
Regulatory bodies such as the U.S. FDA, EMA, and WHO emphasize aseptic manufacturing practices and contamination control in sterile API production. This has prompted major industry players to invest in advanced cleanroom technologies, automated production systems, and digital monitoring tools that ensure consistent product quality and compliance.
Technological Innovations in Manufacturing
Continuous manufacturing, single-use technologies, and advanced filtration systems are transforming sterile API production. These innovations reduce contamination risk, improve scalability, and optimize costs. Integration of automation and AI in quality control processes is further enhancing production efficiency and sterility assurance.
Shift Toward Outsourcing and Global Supply Chain Expansion
Pharmaceutical companies are increasingly outsourcing sterile API manufacturing to specialized contract development and manufacturing organizations (CDMOs) to streamline production and focus on drug innovation. Global CDMOs with sterile facilities are witnessing significant contract inflows from both large pharmaceutical firms and biotech startups, especially across North America, Europe, and Asia-Pacific.
Competitive Landscape
The sterile active pharmaceutical ingredient market is moderately consolidated, with key players focusing on facility expansion, technology integration, and regulatory approvals.
Key Players in the Sterile Active Pharmaceutical Ingredient Market:
- Pfizer CentreOne
- Fresenius Kabi AG
- Siegfried Holding AG
- Hikma Pharmaceuticals PLC
- Lonza Group AG
- Baxter International Inc.
- CordenPharma International
- Dr. Reddy’s Laboratories Ltd.
- Piramal Pharma Limited
- Aurobindo Pharma Limited
These companies are strengthening global supply networks, investing in high-containment manufacturing, and pursuing strategic partnerships to enhance sterile API capabilities.
Recent Developments
- August 2025 – Lonza Group AG announced the expansion of its sterile API manufacturing plant in Switzerland to meet rising biologics production demands.
- March 2025 – Pfizer CentreOne signed a collaboration with a major biotech firm to supply sterile APIs for novel oncology therapeutics.
- October 2024 – Fresenius Kabi launched an advanced aseptic processing line to enhance production efficiency for parenteral formulations.
Regional Insights
North America – Strong Regulatory Oversight and High Biologic Production
The U.S. leads the global market, driven by a robust biopharmaceutical industry, stringent regulatory standards, and the presence of major sterile API manufacturers.
Europe – Expansion in CDMO Capabilities
Countries like Germany, Switzerland, and the U.K. are investing in sterile manufacturing facilities and CDMO partnerships, supported by favorable regulatory environments.
Asia-Pacific – Fastest Growing Market
Emerging economies such as India and China are becoming global sterile API manufacturing hubs, owing to cost-effective production capabilities and rising exports to regulated markets.
Future Outlook: Automation, Single-Use Systems, and Global Integration
The next decade will see sterile API production evolve through:
- AI and Automation: Smart monitoring systems to ensure zero contamination and real-time quality control.
- Single-Use Technologies: Flexible and scalable sterile production with minimized cleaning validation.
- Biologic API Expansion: Rising adoption of large-molecule sterile APIs for targeted therapies.
- Global Integration: Strengthening of international supply chains to support pandemic preparedness and drug availability.
By 2035, the sterile API market will play a crucial role in ensuring global access to safe, effective, and high-quality medicines.