The global dental infection control products market is entering a pivotal decade of innovation and expansion. As dental practices worldwide adopt more rigorous hygiene standards and breakthrough technologies, the sector is poised for sustained growth — transforming from traditional sterilization methods to next‑generation infection prevention systems. This comprehensive forecast outlines key drivers, emerging technologies, regulatory dynamics, and revenue trajectories shaping the market through 2026‑2036.
Market Size & Forecast (2026‑2036)
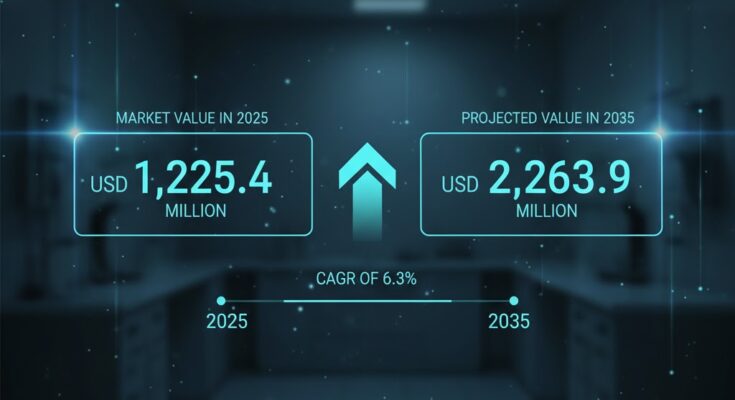
The dental infection control products market, valued at approximately USD 1,225.4 million in 2025, is projected to expand at a 6.3% CAGR to reach around USD 2,263.9 million by 2035. This near‑doubling of revenue highlights the enduring demand for infection control tools, driven by clinical safety priorities and broader healthcare quality expectations. The forecast period to 2036 is expected to reflect similar momentum, with growth supported by both developed and emerging markets investing in advanced sterilization systems, consumables, and digital infection control solutions.
Innovation Catalysts: Driving Next‑Gen Products
1. Smart Sterilization & Automation
One of the most transformative trends in infection control technology is the integration of automation, IoT, and AI into sterilization equipment. Smart autoclaves, sensor‑based monitoring systems, and remote traceability platforms allow clinics to enhance compliance, reduce manual errors, and deliver measurable process assurance in infection prevention.
Digital monitors and connectivity features also support audit trails essential for compliance with regulatory standards, lessening administrative burdens for dental practice managers while raising overall hygiene standards.
2. Eco‑Friendly & Sustainable Solutions
Next‑generation infection control products increasingly emphasize sustainability and eco-sensitive design. Manufacturers are developing biodegradable disinfectants, recyclable packaging for consumables, and energy‑efficient equipment. This reflects growing environmental consciousness among practitioners and aligns with global initiatives to reduce medical waste without compromising clinical safety.
Emerging biodegradable PPE and cleaning compounds are not only compliant with hygiene mandates but also support environmental stewardship — an increasingly important value proposition for modern healthcare facilities.
3. Enhanced Personal Protective & Surface Disinfection Technologies
Improvements in personal protective equipment (PPE) and disinfection technologies are redefining infection control norms in dental settings. This includes high‑filtration masks, anti‑fog shields, advanced gloves, and rapid‑action surface disinfectants with prolonged antimicrobial effects. Touchless devices such as UV‑C disinfection systems and fogging units further minimize manual cleaning gaps, delivering deeper, consistent sanitization.
These innovations are particularly critical in high‑throughput clinics with high patient turnover, where efficient infection prevention directly influences patient safety and practice reputation.
4. Point‑of‑Care Infection Monitoring
Emerging handheld and point‑of‑care microbial detection technologies allow practitioners to rapidly assess contamination risks on surfaces or instruments — often in real time. This locates infection risks before they manifest into clinical issues, enabling proactive intervention rather than reactive cleaning.
Such tools bolster clinician confidence, particularly in environments with immunocompromised patients or complex procedures.
Regulatory Impact: Standards, Compliance & Market Adoption
Regulatory frameworks exert significant influence across the dental infection control landscape. Bodies worldwide maintain stringent infection control guidelines that compel adoption of certified products.
A few key regulatory impacts include:
-
Mandatory sterilization protocols for dental tools and workspaces.
-
Periodic auditing and documentation of infection control compliance.
-
Certification requirements for disinfectants and sterilization systems.
-
Hazard communication and PPE standards designed to protect clinical staff and patients.
These regulations are particularly influential in developed healthcare markets, where compliance costs are accompanied by sophisticated reimbursement systems and higher technology adoption rates. In contrast, emerging markets face both challenges and opportunities due to inconsistent protocol harmonization and varying resource capabilities.
Market Dynamics & Growth Drivers
Increasing Dental Procedures & Oral Disease Prevalence
The rising global incidence of oral health disorders — including caries and periodontal disease — expands procedural volumes across dental practices. This, in turn, escalates demand for infection control consumables (gloves, masks, surface disinfectants) and equipment essential for protecting both patients and providers.
Heightened Awareness Post‑Pandemic
The COVID‑19 pandemic notably heightened awareness of infection risks, leading to a long-term increase in infection control standards across dental settings. Clinics globally have recalibrated practices to include robust sterilization steps previously limited to high-risk environments, embedding infection control deeper into everyday clinical protocols.
Healthcare Infrastructure & Dental Tourism Expansion
Growth in dental tourism and healthcare infrastructure investments across Asia‑Pacific, Latin America, and the Middle East is driving broader uptake of high-quality infection control systems even in previously underserved regions.
Browse Full Report : https://www.factmr.com/report/5378/dental-infection-control-products-market
Regional Market Trends
-
North America: Dominates market revenue due to advanced healthcare infrastructure, early adoption of smart technologies, and stringent regulatory compliance.
-
Europe: Focuses on sustainable product adoption coupled with digital auditing and traceability practices.
-
Asia‑Pacific: Expected to be among the fastest-growing regions, driven by expanding dental networks, rising dental tourism, and increased awareness of infection control importance.
-
Latin America & MEA: Growth supported by infrastructure investments and gradual harmonization of infection control regulations.
Future Outlook: 2030‑2036
Looking ahead, the 2026‑2036 period will likely see:
-
Sharper focus on digital infection control ecosystems, including AI diagnostics and automated compliance tracking.
-
Greater sustainability integration, with reusable and lower-environmental-impact solutions gaining preference.
-
Broader global harmonization of infection control standards, reducing disparities in product adoption between regions.
-
Increased M&A activity and strategic partnerships as leading dental suppliers converge on next-generation product portfolios.
Collectively, these trends indicate not just incremental product evolution but a holistic transformation toward smarter, safer, and more sustainable infection control in dental care.