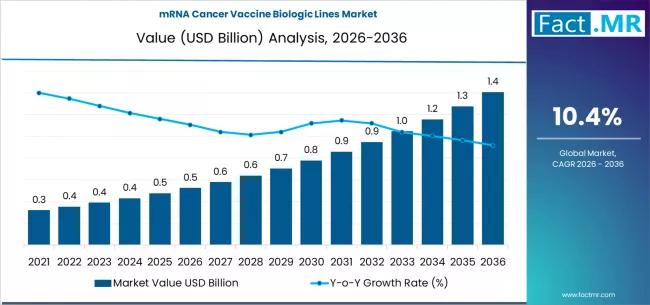
The field of oncology is witnessing a transformative shift as immunotherapy and personalized medicine gain prominence. Among the most promising innovations are mRNA cancer vaccines, which leverage messenger RNA technology to stimulate the body’s immune system to recognize and target cancer cells. The development of mRNA cancer vaccine biologic lines represents a significant advancement in biopharmaceutical research, offering the potential to treat a wide array of malignancies with precision and reduced systemic side effects. As research, clinical trials, and commercial development intensify, the mRNA cancer vaccine biologic lines market is emerging as a critical sector within global oncology therapeutics.
Market Overview
mRNA cancer vaccine biologic lines are designed to encode tumor-specific antigens that trigger targeted immune responses against malignant cells. Unlike traditional vaccines or chemotherapy, these vaccines can be customized to match the unique genetic and molecular profile of a patient’s tumor, enabling a highly personalized therapeutic approach. The biologic lines include the production of stable mRNA constructs, delivery systems such as lipid nanoparticles, and adjunct compounds that enhance immune activation.
The market encompasses pipelines in preclinical research, clinical trials, and commercial-stage biologics targeting solid tumors, hematologic malignancies, and rare cancers. Pharmaceutical and biotech companies are increasingly investing in mRNA technology due to its adaptability, scalable manufacturing potential, and proven success in infectious disease vaccines, which has accelerated its translation into oncology applications.
Key Market Drivers
The growing incidence of cancer worldwide and the need for more effective, personalized treatments are primary drivers of the mRNA cancer vaccine biologic lines market. Traditional therapies, such as chemotherapy and radiation, often cause systemic toxicity and limited efficacy against heterogeneous tumor populations, creating a pressing demand for targeted immunotherapies.
Scientific advancements in genomics, proteomics, and tumor antigen discovery have made it feasible to develop mRNA constructs that elicit robust anti-tumor immunity. Additionally, successful clinical trials demonstrating the safety and efficacy of mRNA vaccines in oncology have bolstered investor confidence and prompted expansion of research pipelines.
Government initiatives, regulatory support for innovative therapies, and increased funding for cancer research further accelerate market development. Collaborations between biotech startups and large pharmaceutical companies are driving innovation and enhancing the speed of commercialization for these biologic lines.
Regional Insights
North America dominates the mRNA cancer vaccine biologic lines market due to the presence of leading biopharmaceutical companies, advanced healthcare infrastructure, and strong investment in oncology research. The region’s regulatory environment and emphasis on innovative therapies support rapid adoption and development of mRNA-based cancer vaccines.
Europe is also a significant market, driven by robust clinical research networks, stringent cancer treatment guidelines, and growing public and private funding for advanced therapeutics. The region emphasizes personalized medicine and precision oncology, which aligns with the capabilities of mRNA cancer vaccines.
Asia-Pacific is emerging as a promising market as countries increase healthcare spending, expand clinical trial capabilities, and foster biotechnology innovation. Rising cancer prevalence, government incentives, and partnerships with global biotech firms are expected to accelerate adoption in this region.
Key Trends & Forecast
Several trends are shaping the mRNA cancer vaccine biologic lines market:
- Personalized and neoantigen-targeted vaccines: Customization of mRNA vaccines based on patient-specific tumor profiles enhances efficacy and minimizes adverse effects.
- Integration with immunotherapy: mRNA vaccines are increasingly being combined with checkpoint inhibitors, CAR-T therapies, and other immunomodulatory treatments to improve outcomes.
- Advancements in delivery systems: Lipid nanoparticles, polymer-based carriers, and novel encapsulation techniques improve mRNA stability and enhance cellular uptake.
- Expansion of clinical pipelines: Companies are accelerating development of vaccines targeting multiple tumor types, including hard-to-treat cancers.
- Regulatory acceleration pathways: Agencies are providing expedited review processes for innovative biologics, fostering faster market entry.
These trends indicate a market that is both innovation-driven and highly responsive to the evolving needs of oncology therapeutics.
Applications & End-Use Outlook
The primary application of mRNA cancer vaccine biologic lines is in personalized oncology, where vaccines are tailored to individual patient profiles. These vaccines target solid tumors such as melanoma, breast cancer, and lung cancer, as well as hematologic malignancies including leukemia and lymphoma.
Clinical research also emphasizes prophylactic and adjuvant use, where vaccines are administered post-surgery or in combination with other therapies to prevent cancer recurrence. Additionally, mRNA vaccines are being explored for rare and difficult-to-treat cancers, expanding the scope of applications beyond conventional therapeutic strategies.
The adoption of mRNA vaccines extends to academic research institutions, biotechnology startups, and pharmaceutical companies developing next-generation immunotherapies. This multi-sector engagement fosters innovation, collaboration, and accelerated clinical development.
Competitive Landscape
The mRNA cancer vaccine biologic lines market is highly competitive, characterized by extensive research pipelines, strategic collaborations, and significant investment in R&D. Key players are leveraging partnerships with academic institutions, contract research organizations, and technology providers to accelerate the development of robust biologic lines.
Innovation in antigen identification, mRNA stabilization, and delivery platforms is a major differentiator in the market. Companies are focusing on pipeline expansion, patent protection, and regulatory alignment to secure competitive advantages. Emerging startups with specialized technology platforms are also influencing market dynamics through innovation and niche applications.
Conclusion
mRNA cancer vaccine biologic lines represent a paradigm shift in oncology, offering personalized, targeted, and effective solutions to combat cancer. By integrating advances in genomics, immunology, and biopharmaceutical engineering, these biologics have the potential to transform cancer treatment and improve patient outcomes. As research and commercialization accelerate, the market for mRNA cancer vaccine biologic lines is poised for growth, providing opportunities for pharmaceutical companies, biotech innovators, and healthcare stakeholders to contribute to the future of precision oncology.
Browse Full Report – https://www.factmr.com/report/mrna-cancer-vaccine-biologic-lines-market