Risk Assessment, Supply Chain Insights & Market Trajectory
The global pelvic floor electrical stimulation devices market is poised for robust expansion through 2036, driven by demographic trends, rising prevalence of pelvic floor disorders, rapid technological innovation, and evolving care delivery models. The market is projected to grow from approximately USD 678.2 million in 2025 to USD 1,470.6 million by 2035, representing a CAGR of ~8.1% over the forecast period.
1. Market Overview & Growth Drivers
Pelvic floor electrical stimulation (PFES) devices are therapeutic technologies designed to strengthen pelvic floor muscles using controlled electrical pulses. They provide non‑invasive treatment for conditions such as urinary incontinence, fecal incontinence, pelvic organ prolapse, and certain forms of sexual dysfunction. These devices are increasingly integrated into urogynecology and physiotherapy care pathways as first‑line or adjunct treatments, particularly in older adults and post‑partum women.
The primary drivers of market growth include:
-
Increasing Prevalence of Pelvic Floor Disorders: Rising incidence of urinary incontinence and pelvic dysfunction, particularly among aging populations and postpartum women, is significantly driving demand. Incontinence remains among the largest application segments, capturing a substantial share of annual device utilization in many mature markets.
-
Shift to Non‑Surgical Therapies: Healthcare systems and clinicians favor non‑invasive, safe alternatives to surgical interventions due to lower risk profiles, reduced recovery times, and enhanced patient comfort. PFES devices are emerging as key elements of conservative therapy regimens for mild to moderate cases.
-
Technological Innovation: Product innovation — including wireless connectivity, smartphone integration, remote monitoring, AI‑driven personalization, and wearable formats — is expanding access and adherence by facilitating home-based therapy and real-time tracking.
-
Supportive Reimbursement Policies: Evolving reimbursement frameworks in North America and Europe are lowering financial barriers by including PFES devices and related therapies under health insurance coverage, especially where clinical guidelines emphasize conservative management.
2. Supply Chain & Technology Adoption Dynamics
The PFES market’s supply chain and technology landscape are shaped by multiple intersecting trends:
-
Digitization & Connectivity: Manufacturers increasingly emphasize digital health capabilities — such as Bluetooth, app control, and cloud analytics — enabling remote therapy and integration with electronic health records. This supports clinician oversight and enhances patient engagement through personalized feedback loops.
-
Biofeedback & AI: Next-generation systems incorporate biofeedback and AI-based therapy customization to optimize treatment regimens, track progress, and tailor stimulation protocols to individual patient profiles. These advancements expand clinical utility beyond basic electrical stimulation.
-
Wearables & Homecare Devices: The trend towards mobile and wearable devices is accelerating, with compact, discreet units designed for home therapy capturing a growing share of sales. This enhances accessibility for patients with mobility constraints or privacy concerns.
-
Clinical Evidence & Education: Strengthening clinical evidence through trials and post-market studies helps validate efficacy, support guideline inclusion, and reduce clinician hesitation. Industry initiatives to educate healthcare providers, particularly in physiotherapy and urogynecology, are improving adoption rates.
3. Regional Trajectory & Segment Performance
The geographic landscape of the PFES devices market reveals differentiated growth drivers and adoption patterns:
-
North America: High healthcare spending, advanced clinical infrastructure, and strong insurance coverage support a healthy growth rate. Early adoption of digital devices and extensive clinician training programs reinforce leadership in this region.
-
Europe: Market growth is catalyzed by sustainability trends, national health programs targeting women’s health, and regulatory clarity. Home-use solutions and remote monitoring are gaining traction across major EU markets.
-
Asia‑Pacific: Emerging economies including China, India, Japan, and South Korea are rapidly expanding, fueled by increasing healthcare access, public health initiatives, rising awareness, and urban middle-class demand for non-invasive therapies. Government programs on maternal and women’s health further bolster market penetration.
-
Middle East & Africa: While currently smaller in absolute terms, this region is beginning to gain momentum as private healthcare reforms, rising awareness, and pilot pelvic health programs increase adoption, particularly in urban centers.
Segment insights further emphasize market dynamics:
-
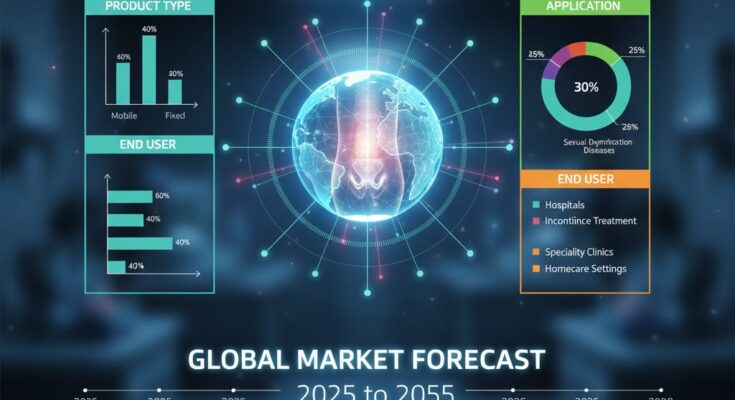
By Product Type: Mobile and wearable PFES devices capture the majority of market share due to convenience, ease of use, and home therapy trends, while fixed clinical systems remain important in hospital and specialized settings.
-
By Application: Urinary incontinence dominates application usage, followed by significant demand for devices treating aspects of sexual dysfunction and other pelvic floor disorders.
4. Risk Assessment & Market Challenges
Despite strong growth prospects, the PFES market faces several risks and constraints:
-
Regulatory Complexity: Medical device approval pathways vary globally, creating compliance challenges, especially in emerging markets with evolving regulatory frameworks. Differences in classification, testing requirements, and import controls can delay market entry and increase costs.
-
Technological Barriers: Adoption of advanced technologies — such as AI and real-time connectivity — requires investment in software development and cybersecurity measures, which may increase production costs and raise data privacy concerns.
-
Affordability & Insurance Gaps: High device costs and limited insurance coverage in some regions restrict access for lower-income and rural populations, dampening market potential. Affordability concerns remain prominent where reimbursement policies are nascent.
-
Awareness & Stigma: In certain cultures, pelvic floor disorders carry social stigma, leading to under-reporting and delayed treatment seeking, which constrains early diagnosis and uptake of PFES solutions.
Browse Full Report : https://www.factmr.com/report/5245/pelvic-floor-electrical-stimulation-devices-market
5. Market Trajectory: 2026–2036
Looking ahead to 2026–2036, the market trajectory is anchored on:
-
Sustained CAGR Growth: Continued double-digit adoption in select subsegments — notably wearables and app-integrated systems — will reinforce long-term growth.
-
Ecosystem Partnerships: Strategic alliances between device manufacturers, digital health platforms, and insurers will accelerate access and reimbursement strategies worldwide.
-
Enhanced Patient Care Models: Expansion of telemedicine, remote monitoring, and care coordination will integrate PFES into broader chronic care management, especially for aging populations.
In summary, the pelvic floor electrical stimulation devices market is transitioning from niche clinical use toward mainstream therapeutic adoption, driven by technological evolution, demographic pressure, healthcare system support, and an expanding base of clinical evidence. Manufacturers that strategically navigate regulatory challenges, supply chain complexities, and localized awareness campaigns will sustain competitive advantage in the decade ahead.