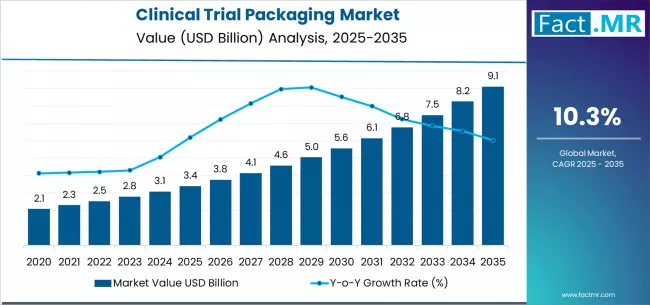
The global clinical trial packaging market is poised for significant growth in the coming years, fueled by the increasing number of clinical trials worldwide, stringent regulatory requirements, and advancements in packaging technologies. The industry is projected to reach USD 6.8 billion by 2030, growing at a CAGR of 7.2% from 2025 to 2030, reflecting strong demand across pharmaceutical, biotech, and contract research organizations (CROs).
Clinical trial packaging plays a critical role in ensuring the safety, integrity, and compliance of investigational drugs. Market growth is driven by the rising prevalence of chronic diseases, expansion of biopharmaceutical research, and the ongoing need for innovative packaging solutions to support complex clinical trial protocols.
Key Market Insights:
-
Rising Clinical Trials: The number of clinical trials globally has surged in the past decade, marking a significant increase from previous years. This growth directly translates into higher demand for specialized packaging solutions that can maintain drug stability, support temperature-sensitive products, and ensure compliance with regulatory standards.
-
Regulatory Compliance Driving Innovation: Regulatory authorities have emphasized stringent packaging and labeling requirements for investigational drugs. This has accelerated adoption of tamper-evident, child-resistant, and serialization-enabled packaging solutions, ensuring both patient safety and traceability throughout the clinical trial process.
-
Market Segmentation:
-
By Packaging Type: Blister packs and bottles dominate the market, together accounting for over 60% of global revenue in 2024. Advanced pre-filled syringes and vials are witnessing rapid adoption in biologics trials.
-
By Material: Plastic-based packaging held 55% of the market share in 2024 due to its lightweight and cost-effective properties, while glass packaging remains preferred for high-potency drugs.
-
By End-User: Pharmaceutical companies account for nearly 70% of the demand, with CROs and biotech firms driving the remainder.
-
-
Regional Analysis:
-
North America leads the market, contributing approximately 40% of global revenue, driven by high clinical trial activity and strong regulatory oversight.
-
Europe follows closely, with increasing adoption of sustainable and child-resistant packaging solutions.
-
Asia-Pacific is expected to witness the fastest growth at a CAGR of 8.1%, driven by rising clinical research investments in emerging economies.
-
-
Technological Advancements: Smart packaging solutions, such as temperature-monitoring labels, RFID-enabled tracking, and digital dose monitoring, are gaining traction. These innovations enhance patient safety and ensure compliance with clinical practice guidelines.
Industry Drivers and Opportunities:
-
Expanding Biopharmaceutical Pipeline: Biologics, gene therapies, and personalized medicines require advanced packaging solutions to maintain stability, creating a high-growth niche in the clinical trial packaging segment.
-
Outsourcing Trend: Pharmaceutical companies increasingly rely on CROs and contract packaging organizations to manage complex trial logistics, offering significant growth opportunities for service providers.
-
Sustainability Focus: Regulatory push towards eco-friendly, recyclable, and biodegradable packaging materials is creating new opportunities for innovation and differentiation in the market.
Challenges:
Despite strong growth prospects, the market faces challenges such as high initial investment costs, stringent regulatory hurdles, and supply chain disruptions due to global material shortages. Strategic partnerships and technological innovation are mitigating these risks.
Leading Players and Competitive Landscape:
The clinical trial packaging market is highly competitive, with major players focusing on product innovation, strategic collaborations, and expansion into emerging markets. Key companies include:
-
Catalent, Inc. – Offering advanced drug delivery and clinical supply services.
-
PCI Pharma Services – Specializing in packaging solutions for biologics and orphan drugs.
-
Sharp Packaging Services – Known for automated clinical packaging and serialization capabilities.
-
Feldan Therapeutics – Focused on temperature-sensitive and complex drug packaging solutions.
These companies are investing heavily in R&D, smart packaging technologies, and sustainable materials to capture growing market opportunities.
Browse Full Report : https://www.factmr.com/report/clinical-trial-packaging-market
Expert Insights:
“Clinical trial packaging is no longer just about containment; it is an integral part of clinical trial design and patient safety,” said an industry analyst. “With increasing regulatory scrutiny and technological innovation, the market is poised for robust growth, especially in biologics and complex therapies.”
Conclusion:
The global clinical trial packaging market is entering a transformative phase, driven by rising clinical trials, technological innovation, and regulatory requirements. With an expected valuation of USD 6.8 billion by 2030 and a CAGR of 7.2%, the market presents lucrative opportunities for manufacturers, CROs, and pharmaceutical companies. Sustainable and intelligent packaging solutions will play a pivotal role in enhancing patient safety, regulatory compliance, and operational efficiency.