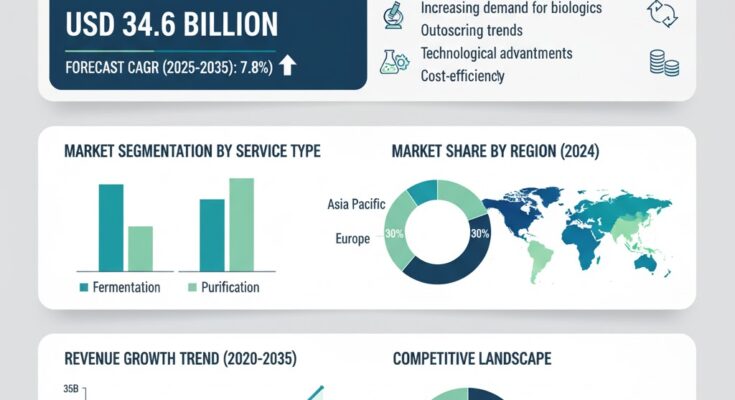
The global contract pharmaceutical fermentation services market is on a strong growth trajectory, driven by increasing demand for outsourced biopharmaceutical manufacturing, rising adoption of advanced fermentation technologies, and the growing need for scalable production solutions. Valued at USD 16.0 billion in 2025, the market is projected to reach USD 34.6 billion by 2035, recording an absolute increase of USD 18.6 billion and expanding at a robust CAGR of 7.8% during the forecast period.
As pharmaceutical and biotechnology companies focus on cost-effective production, time-to-market reduction, and specialized expertise, contract fermentation service providers are becoming indispensable partners. These services offer high-quality, scalable manufacturing solutions for microbial, mammalian, and recombinant protein-based therapeutics, positioning them as a critical component of the modern drug development pipeline.
Market Drivers: Outsourced Manufacturing, Biopharma Growth, and Technological Advancements
Rising Demand for Outsourced Biopharmaceutical Production
The increasing complexity of biologics and biosimilars is pushing pharmaceutical firms to outsource fermentation processes to specialized contract service providers. This trend allows companies to leverage advanced infrastructure, regulatory expertise, and process optimization without significant capital investment.
Technological Innovation and Scale-Up Capabilities
Advancements in microbial and mammalian fermentation technologies, including high-density cell cultures, automated bioreactors, and continuous fermentation platforms, are improving yield, consistency, and process efficiency. Integration with digital monitoring, AI-driven process control, and real-time analytics further enhances production outcomes, enabling high-quality therapeutics at reduced costs.
Strategic Shift Toward Flexible and Personalized Manufacturing
Healthcare companies are increasingly adopting personalized medicine approaches, requiring flexible, small- to large-scale fermentation solutions. Contract services provide the agility to meet varying batch sizes, rapid scale-up requirements, and strict regulatory compliance, supporting timely delivery of novel therapies to patients worldwide.
Competitive Landscape
The contract pharmaceutical fermentation services market is highly competitive, with leading players focusing on capacity expansion, advanced technology adoption, regulatory compliance, and global market reach.
Key Players in the Market Include:
- Lonza Group AG
- Thermo Fisher Scientific Inc. (Patheon)
- Fujifilm Diosynth Biotechnologies
- Samsung Biologics Co., Ltd.
- Catalent, Inc.
- Boehringer Ingelheim GmbH
- WuXi Biologics (Cayman) Inc.
- AGC Biologics
- Novasep Holding SAS
- Rentschler Biopharma SE
These companies are strengthening their pipelines, enhancing bioreactor capacities, and forging strategic collaborations with pharmaceutical innovators to accelerate adoption. Key strategies include regulatory approvals, acquisitions, and partnerships with biotech startups.
Recent Developments
- 2025 – Thermo Fisher Scientific expanded its microbial fermentation capacity in Europe to support increasing demand for biosimilars and specialty biologics.
- 2024 – Lonza Group AG inaugurated a new mammalian cell culture facility in the U.S., enabling large-scale production of monoclonal antibodies and gene therapy products.
Future Outlook: Smarter and Scalable Manufacturing Solutions
Over the next decade, contract fermentation services will evolve with:
- AI-Driven Process Optimization – Predictive analytics for improved yield and efficiency.
- Flexible Biomanufacturing – Rapid scale-up capabilities for personalized medicine.
- Sustainable Production – Eco-friendly bioprocessing methods and energy-efficient operations.
- Global Accessibility – Increased presence in emerging markets to meet growing therapeutic demand.
By 2035, the contract pharmaceutical fermentation services market will play a pivotal role in the biopharmaceutical industry, providing advanced, scalable, and patient-centric manufacturing solutions.