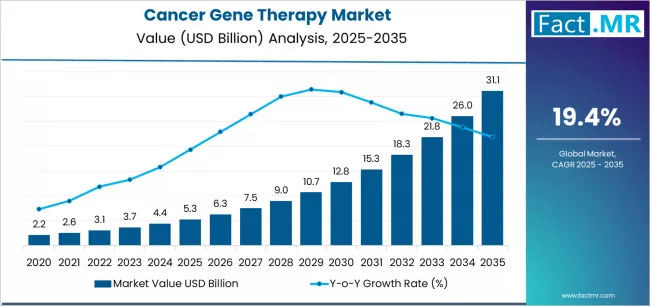
Cancer gene therapy is revolutionizing oncology by offering targeted, personalized treatments that modify patients’ genetic material to combat malignancies. Unlike conventional therapies such as chemotherapy and radiation, gene therapy focuses on correcting or altering disease-causing genes, enabling precise eradication of cancer cells while minimizing damage to healthy tissues. With advancements in CAR-T cell therapies, viral vectors, and gene-editing technologies, the cancer gene therapy market is becoming a critical driver of innovation in hematologic and solid tumor treatment.
Market Overview:
Cancer gene therapy encompasses a range of approaches, including ex vivo and in vivo therapies, viral and non-viral vector delivery systems, and engineered immune cell treatments. CAR-T cell therapy, for example, involves modifying a patient’s T-cells to target cancer-specific antigens, providing durable responses even in treatment-refractory cases. Oncolytic viruses and gene-modified cellular immunotherapies are increasingly used to stimulate anti-tumor immunity and achieve sustained disease control. These therapies offer patients the potential for complete remission and improved survival, transforming the landscape of cancer care.
Key Segments:
The market is segmented by cancer indication, vector type, and route of administration. Large B-cell lymphoma dominates the therapeutic landscape due to multiple FDA-approved CAR-T therapies demonstrating high efficacy in refractory diffuse large B-cell lymphoma and follicular lymphoma populations. Multiple myeloma is also a growing segment, driven by BCMA-targeted CAR-T therapies and clinical evidence supporting their use in plasma cell malignancies. Vector technologies such as lentivirus lead the market because of high transduction efficiency and stable gene integration, essential for ex vivo modification of immune cells. Intravenous administration remains the primary delivery route, particularly for systemic treatment of hematologic cancers.
Regional Insights:
North America leads the cancer gene therapy market, driven by pioneering CAR-T development, advanced cancer treatment centers, and comprehensive reimbursement infrastructure. Europe follows closely, emphasizing evidence-based adoption and health technology assessment frameworks. Asia-Pacific is emerging as a high-growth region due to domestic CAR-T approvals, expanding clinical trial infrastructure, and increasing investments in cellular immunotherapy programs. Latin America and the Middle East & Africa are gradually adopting gene therapy technologies, supported by growing awareness of innovative oncology treatments and improving healthcare infrastructure.
Trends & Drivers:
The market is propelled by rising demand for precision medicine and breakthrough therapies for treatment-resistant cancers. Patients facing limited options after multiple chemotherapy regimens are increasingly seeking gene therapy solutions that offer durable responses and potential curative outcomes. The expansion of CAR-T therapy approvals and clinical validation of gene-modified cellular immunotherapies drives both patient access and adoption by healthcare institutions. Investments in viral vector manufacturing and cell processing infrastructure support large-scale production, ensuring consistent availability of high-quality therapies.
Additional trends include the development of next-generation CAR-T constructs with enhanced safety profiles, CRISPR-enabled gene editing for improved immune cell functionality, and off-the-shelf allogeneic therapies that reduce manufacturing time and cost. Integration of digital health and bioinformatics platforms also allows clinicians to better select patients, optimize dosing, and monitor therapeutic outcomes.
Challenges & Barriers:
Despite its transformative potential, the cancer gene therapy market faces challenges. High treatment costs, risk of severe side effects such as cytokine release syndrome, and the need for specialized healthcare facilities limit widespread adoption. Complex regulatory pathways and rigorous clinical validation requirements can slow therapy commercialization. Furthermore, maintaining equitable access to cutting-edge therapies across regions with varying healthcare infrastructure remains a key concern for stakeholders.
Applications & End-Use Outlook:
Cancer gene therapies are primarily administered in specialized academic medical centers, National Cancer Institute-designated facilities, and authorized treatment centers equipped with apheresis and cell processing capabilities. Hematology-oncology specialists and multidisciplinary care teams play a crucial role in patient selection, treatment administration, and post-therapy monitoring. The therapies address hematologic malignancies including large B-cell lymphoma, multiple myeloma, and acute lymphoblastic leukemia, with emerging applications in solid tumors through targeted CAR-T and oncolytic virus therapies. Hospitals, research institutes, and clinical trial centers also contribute to market expansion by facilitating patient access and supporting ongoing innovation.
Future Outlook:
The cancer gene therapy market is poised for substantial growth as technology advances, clinical evidence accumulates, and regulatory pathways evolve to support innovative therapies. Next-generation CAR-T products, gene-edited immune cells, and allogeneic universal donor therapies are expected to broaden treatment options and improve patient outcomes. Strategic collaborations between pharmaceutical companies, biotechnology firms, and academic institutions will continue to accelerate innovation, while decentralized manufacturing and outpatient delivery models increase patient accessibility.
As precision oncology gains traction, gene therapy is likely to become a standard component of treatment regimens, offering long-term disease control, functional cures, and improved quality of life. Expanding pipeline activity, with hundreds of ongoing clinical trials, signals significant opportunities for new indications, technological advancements, and global adoption.
Conclusion:
Cancer gene therapy represents a paradigm shift in oncology, delivering highly targeted, potentially curative treatments for patients with treatment-refractory and aggressive cancers. Through advancements in CAR-T therapy, viral vectors, and gene editing, the market is redefining cancer treatment by enhancing efficacy, safety, and personalization. With continued innovation, strategic collaborations, and expanding global infrastructure, cancer gene therapy is poised to transform the future of cancer care, offering hope and improved outcomes for patients worldwide.
Browse Full Report – https://www.factmr.com/report/cancer-gene-therapy-market