Global Human Platelet Lysate Market Set for Decisive Growth (2026–2036) as Regenerative Medicine Shifts Toward Xeno-Free Standards
The global Human Platelet Lysate (HPL) market is entering a transformative decade, with industry forecasts for 2026–2036 signaling a robust transition toward standardized, clinical-grade cell culture supplements. As the biopharmaceutical sector aggressively pivots away from animal-derived components like Fetal Bovine Serum (FBS), HPL has emerged as the gold standard for the safe and efficient expansion of mesenchymal stem cells (MSCs) and other therapeutic cell lines.
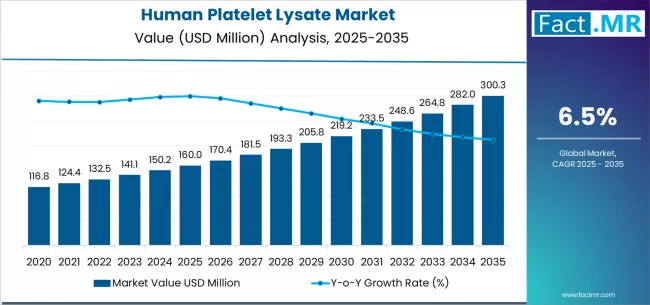
Recent market intelligence indicates that the HPL industry, valued at approximately $677 million in 2026, is projected to achieve a compound annual growth rate (CAGR) of over 15%, potentially exceeding $2.5 billion by 2036. This surge is underpinned by a global rise in regenerative medicine trials and the industrialization of cell and gene therapies (CGT).
Strategic Market Overview: The Who, What, and Why
-
Who: The market is driven by leading biotechnology firms, including Merck KGaA, STEMCELL Technologies, PL BioScience, and Mill Creek Life Sciences, alongside specialized Contract Development and Manufacturing Organizations (CDMOs).
-
What: Human Platelet Lysate is a growth-factor-rich supplement derived from donor platelets. It serves as a high-performance, non-animal alternative to traditional sera.
-
When: The forecast period of 2026–2036 marks the industrialization phase of HPL, where production moves from small-batch research use to large-scale, GMP-compliant manufacturing.
-
Where: While North America currently holds the largest market share (approx. 41%), the Asia-Pacific region is expected to witness the highest growth rate due to surging investments in stem cell research in China, India, and South Korea.
-
Why: Increasing regulatory pressure to eliminate zoonotic risk and batch-to-batch variability in clinical manufacturing has made HPL indispensable for FDA and EMA-compliant workflows.
The Rise of Heparin-Free and Pathogen-Inactivated Formulations
A critical trend shaping the 2026–2036 landscape is the dominance of heparin-free human platelet lysate. Traditionally, HPL required the addition of porcine-derived heparin to prevent coagulation during cell culture. However, the industry is rapidly adopting second-generation, fibrinogen-depleted heparin-free versions.
This segment now accounts for over 60% of new clinical projects, as it offers a more defined environment and further reduces animal-origin risks. Furthermore, pathogen inactivation (PI) technologies are becoming a standard requirement for allogeneic therapies, ensuring that pooled donor materials meet the highest safety profiles for human injection.
Key Market Drivers & Data-Backed Insights
-
Clinical Trial Volume: With over 1,200 cell therapy clinical trials active globally as of 2026, the demand for scalable ancillary materials has never been higher.
-
Chronic Disease Prevalence: The rising incidence of orthopedic disorders, autoimmune diseases, and neurodegenerative conditions is fueling the demand for MSC-based therapies, which rely heavily on HPL for rapid cell doubling.
-
Ethical & Regulatory Shift: Global regulatory bodies are increasingly advocating for xeno-free (animal-free) manufacturing environments. HPL provides the essential cytokines—such as PDGF, TGF-β, and EGF—necessary for cell health without the ethical or safety concerns of bovine products.
Regional Outlook and Sector Potential
-
North America: Dominance is maintained through a robust ecosystem of biotech giants and favorable regulatory pathways (e.g., RMAT designation).
-
Europe: Strong academic-industrial partnerships in Germany and France are driving innovations in precision lysates tailored for specific cell lineages.
-
Asia-Pacific: Projected to grow at a CAGR of 17%+, driven by government-backed regenerative medicine initiatives and a growing infrastructure for specialized blood component processing.
The shift from research-grade to GMP-grade HPL is no longer a luxury—it is a regulatory necessity, states a leading industry analyst. The 2026-2036 window will see HPL evolve from a niche supplement into a foundational pillar of the global bioprocessing supply chain.
Future Outlook: AI and Automated Manufacturing
Looking toward 2036, the market is expected to integrate Artificial Intelligence (AI) to optimize donor pooling and cytokine profiling. This Precision HPL will allow manufacturers to customize batches for specific therapeutic outcomes, such as enhancing the potency of CAR-T cells or accelerating the maturation of tissue-engineered skin grafts.
About the Report This market summary provides a comprehensive analysis of the Human Platelet Lysate landscape for the 2026–2036 period, offering insights for investors, biopharmaceutical leaders, and clinical researchers navigating the future of regenerative medicine.
Browse Full Report : https://www.factmr.com/report/human-platelet-lysate-market
– Contact Us –
11140 Rockville Pike, Suite 400, Rockville,
MD 20852, United States
Tel: +1 (628) 251-1583 | sales@factmr.com
About Fact.MR
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.