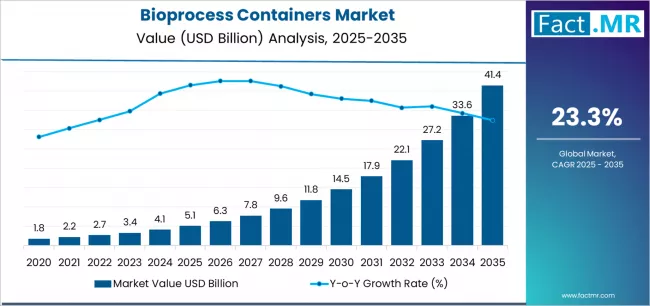
The bioprocess containers market is experiencing significant growth as the biopharmaceutical industry increasingly adopts flexible, single-use manufacturing systems. Bioprocess containers, often integrated with advanced sensor technology, provide reliable solutions for liquid handling, storage, and process monitoring in biologics production. These containers are essential for improving operational efficiency, reducing contamination risks, and supporting scalable, modular manufacturing setups, which are critical for modern biopharmaceutical and cell therapy production platforms.
Market Overview
Bioprocess containers are specialized vessels designed for storing, transporting, and processing liquids in biomanufacturing environments. They are widely used in upstream and downstream bioprocessing, including media preparation, cell culture, buffer storage, and formulation. Their single-use design minimizes contamination risk and reduces the need for complex cleaning and sterilization procedures.
Advancements in container materials and designs, such as 2D and 3D configurations, have enhanced their compatibility with automated and continuous bioprocessing systems. Sensor-integrated containers enable real-time monitoring of critical parameters, ensuring high-quality outcomes and regulatory compliance. The versatility of bioprocess containers makes them indispensable for both large-scale biologics production and specialized cell and gene therapy applications.
Key Drivers of Market Growth
- Rising Biopharmaceutical Production
The growing demand for monoclonal antibodies, cell and gene therapies, and biosimilars is driving the need for advanced bioprocess containers. Manufacturers are adopting single-use technologies to scale operations efficiently while maintaining product quality. - Emphasis on Contamination Prevention
Contamination control is a critical priority in biomanufacturing. Bioprocess containers reduce cross-contamination risks and improve sterility assurance, making them essential for meeting stringent regulatory standards. - Adoption of Single-Use Systems
Single-use containers offer operational flexibility, reduce changeover times, and support multi-product facilities. Their adoption has become a standard practice in modern biomanufacturing, particularly in flexible and modular production environments. - Technological Innovations
Advances in container materials, sensor integration, and design innovation enhance monitoring, scalability, and process control. These innovations improve reliability and compatibility with continuous manufacturing processes. - Expansion of Biomanufacturing Infrastructure
The growth of contract manufacturing organizations (CMOs) and new biopharmaceutical facilities globally has increased the demand for standardized, high-performance bioprocess containers capable of supporting diverse production workflows.
Market Segmentation
By Product Type
- 2D Bioprocess Containers: Widely used for media and buffer storage, these containers are favored for their ease of handling and robust performance in upstream processes.
- 3D Bioreactor Systems: These containers are utilized in advanced cell culture applications, providing scalability, uniform mixing, and compatibility with automated monitoring systems.
By Application
- Upstream Processes: Containers are primarily used in media preparation, cell cultivation, and fermentation applications.
- Downstream Processes: Some containers support filtration, formulation, and storage processes, though upstream applications dominate market usage.
By End-User
The primary end-users include pharmaceutical and biopharmaceutical manufacturers, contract manufacturing organizations, and specialized cell and gene therapy production facilities. These stakeholders prioritize operational reliability, sterility, and regulatory compliance.
Regional Insights
North America
North America remains a leading market due to a well-established biopharmaceutical industry, adoption of advanced single-use systems, and focus on continuous manufacturing innovations.
Europe
Europe emphasizes stringent regulatory compliance, high-quality production standards, and operational efficiency, driving demand for high-performance bioprocess containers in established biologics facilities.
Asia-Pacific
Asia-Pacific is an emerging market, supported by expanding biomanufacturing infrastructure, rising biosimilar production, and increasing investment in modular and flexible facilities. The region is rapidly adopting single-use container technologies to improve scalability and production efficiency.
Other Regions
Emerging markets in Latin America, the Middle East, and Africa are gradually increasing adoption of bioprocess containers as biopharmaceutical manufacturing capabilities expand and global regulatory alignment improves.
Key Trends and Opportunities
Sensor-Integrated Containers
Integration of real-time monitoring sensors enables precise process control, better data collection, and higher product quality, offering opportunities for innovation in container design.
Flexible Manufacturing Systems
Modular and scalable production setups are creating demand for bioprocess containers that can easily adapt to changing production volumes and multi-product manufacturing workflows.
Continuous Manufacturing Adoption
Continuous bioprocessing requires containers that support uninterrupted workflows while maintaining sterility and operational efficiency, providing growth opportunities for advanced container technologies.
Expansion of Cell and Gene Therapy Production
The growth of personalized medicine and cell therapy applications is driving demand for specialized containers capable of supporting sensitive and high-value biologics.
Conclusion
The bioprocess containers market is poised for substantial growth as biopharmaceutical manufacturers prioritize operational flexibility, contamination control, and scalable production. Single-use containers, particularly advanced 2D and 3D systems with sensor integration, are becoming indispensable in modern biomanufacturing environments.
As the industry expands into new therapeutic areas such as cell and gene therapy and biosimilars, the demand for innovative, reliable, and flexible bioprocess container solutions will continue to rise. Manufacturers and contract organizations that invest in advanced container technologies, modular facility integration, and real-time process monitoring will gain a competitive edge, ensuring efficiency, regulatory compliance, and high-quality production outcomes.
The future of bioprocess containers lies in technological innovation, operational flexibility, and the growing adoption of single-use systems, positioning them as a critical component of modern, high-performance biomanufacturing.
Browse Full Report – https://www.factmr.com/report/bioprocess-containers-market