The global market for molecular diagnostics targeting sexually transmitted diseases (STDs) is poised for substantial growth through 2035 as healthcare systems prioritize rapid, accurate, and point-of-care testing to curb infection spread and improve patient outcomes. Molecular methods — including nucleic acid amplification tests (NAATs), PCR-based assays, isothermal amplification, and multiplexed platforms — deliver high sensitivity and specificity for pathogens such as chlamydia, gonorrhea, syphilis (molecular assays for Treponema detection), trichomonas, Mycoplasma genitalium, herpes simplex virus, and HIV viral load monitoring. These technologies are increasingly favored over older serologic or culture-based tests because they enable earlier detection, targeted therapy, and antimicrobial-resistance surveillance.
Expansion in public-health screening programs, growing awareness of asymptomatic infections, increased funding for sexual-health initiatives, and technological advances in sample-to-answer platforms are collectively driving market momentum. Demand is rising across hospitals, specialized STD clinics, community health centers, and decentralized point-of-care settings — including mobile testing units and pharmacy-based services.
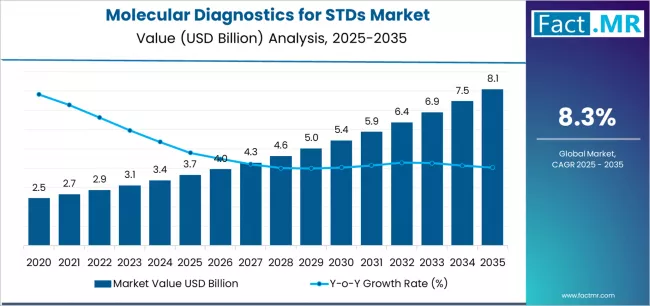
Quick Stats (2025–2035)
-
Market Value 2025: Significant multi-billion-dollar market (global molecular STD diagnostics)
-
Forecast 2035: Strong growth driven by screening expansion, multiplex adoption, and point-of-care deployment
-
Forecast CAGR (2025–2035): Mid-to-high single digits to low double digits, depending on region and adoption of point-of-care platforms
-
Leading Technologies (2025): NAATs / PCR-based assays
-
Fastest-Growing Segment: Multiplex platforms and point-of-care molecular tests
-
Key Growth Regions: North America, Europe, Asia-Pacific, with emerging growth in Latin America and Africa
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=12228
Market Drivers
Rising Prevalence & Screening Initiatives
Many STDs are frequently asymptomatic yet cause long-term health consequences, such as infertility, adverse pregnancy outcomes, and increased HIV transmission risk. Public-health agencies and clinicians are expanding screening guidelines (especially for high-risk populations) which fuels demand for reliable molecular testing that can be deployed in high-throughput labs or near the patient.
Superior Clinical Performance of Molecular Tests
Molecular diagnostics offer greater sensitivity and specificity than traditional methods, reduce time-to-result, and enable testing from non-invasive specimens (urine, self-collected vaginal swabs, rectal or pharyngeal swabs). This performance advantage supports wider clinical adoption and patient acceptance.
Multiplex Testing & Syndromic Panels
Multiplex and syndromic molecular panels that detect multiple pathogens from a single specimen are gaining traction for symptomatic patients and surveillance programs. These panels improve diagnostic yield, streamline clinical workflows, and support antimicrobial stewardship by enabling pathogen-specific therapy.
Point-of-Care & Decentralized Testing
Advances in simplified, cartridge-based molecular platforms enable accurate testing near the patient with rapid turnaround times. Point-of-care molecular tests accelerate diagnosis and treatment decisions during the same clinical encounter, reducing loss-to-follow-up and improving public-health outcomes.
Antimicrobial Resistance (AMR) Surveillance
Rising resistance in pathogens like Neisseria gonorrhoeae has made molecular assays that detect resistance markers or guide susceptibility increasingly important. Molecular resistance testing supports targeted therapy and broader surveillance efforts, particularly in high-burden settings.
Market Structure & Segment Insights
By Test Type
-
Nucleic Acid Amplification Tests (NAATs)/PCR: Dominate the market due to accuracy and regulatory acceptance.
-
Isothermal Amplification & Rapid Molecular Assays: Growing rapidly for decentralized testing due to lower instrument complexity.
-
Multiplex/Syndromic Panels: Increasing adoption in hospitals and reference labs for comprehensive STI workups.
By End-User
-
Hospital and Reference Laboratories: Continue to be the largest users for confirmatory and high-volume testing.
-
Primary Care & Sexual Health Clinics: Growing adoption of near-patient molecular testing to enable faster treatment.
-
Point-of-Care & Community Programs: Expansion in outreach, mobile clinics, and pharmacy testing initiatives to improve accessibility.
By Specimen Type
Self-collected vaginal swabs and urine specimens are driving uptake due to ease of collection and improved screening coverage, especially among populations reluctant to seek clinical sampling.
Regional Dynamics
North America leads in value due to established screening programs, favorable reimbursement environments, and strong adoption of advanced molecular platforms. Europe follows, with emphasis on public-health screening and integrated surveillance. Asia-Pacific is a high-growth region driven by expanding healthcare infrastructure, rising STD awareness, and investments in decentralizing diagnostics. Emerging regions in Latin America and Africa show increasing demand as donor-funded programs and national screening initiatives expand.
Challenges & Restraints
Cost & Reimbursement Barriers
Molecular tests and instruments can be more expensive than traditional methods, and reimbursement variability across regions can limit adoption—particularly in low-resource settings.
Infrastructure & Training Needs
Laboratory capacity, skilled technicians, and quality-assurance frameworks are necessary for reliable molecular testing. Scaling these capabilities in decentralized settings requires investment in training and oversight.
Regulatory & Quality Standards
Approvals and performance validation for multiplex or resistance assays vary by jurisdiction, and harmonizing standards remains an ongoing challenge.
Specimen Access & Stigma
Social stigma and barriers to testing can limit specimen collection; however, innovations such as self-collection kits and community-based testing help address these issues.
Opportunities & Future Trends
Expansion of Self-Collection & Home Testing
Validated self-collection kits coupled with laboratory-based molecular testing or mail-in options can increase screening uptake and early detection.
Integrated Digital Reporting & Surveillance
Connectivity between molecular platforms and public-health reporting systems enhances real-time surveillance and outbreak tracking, enabling faster public-health responses.
Combined STD/HIV Testing Workflows
Integrated testing pathways and panels that include HIV viral load or antigen testing alongside common STIs streamline care and improve linkage to treatment.
AMR-Guided Molecular Assays
Molecular assays that detect resistance markers will become critical in clinical decision support and stewardship programs, especially for gonorrhea management.
Outlook
The molecular diagnostics market for sexually transmitted diseases is set for sustained growth through 2035 as health systems emphasize rapid, accurate testing, decentralize diagnostics, and integrate resistance surveillance. Companies that deliver affordable, easy-to-use molecular platforms, support self-collection pathways, and provide integrated data connectivity will be well-positioned to capture expanding public-health and clinical testing demand worldwide. Improved access to testing, combined with targeted treatment enabled by molecular insights, promises to reduce STD burden and improve reproductive and public-health outcomes globally.
Browse Full Report: https://www.factmr.com/connectus/sample?flag=S&rep_id=12228