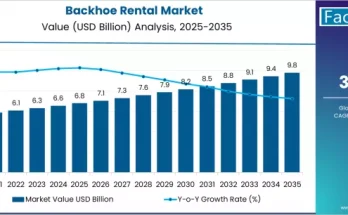
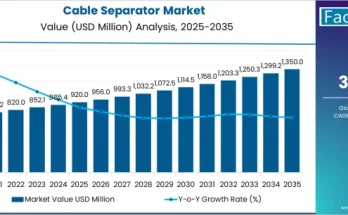
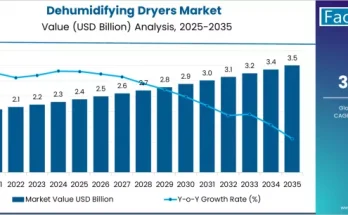
The pharmaceutical industry in the United Kingdom is experiencing rapid evolution driven by rising healthcare needs, regulatory scrutiny, and growing reliance on complex drug formulations. Active Pharmaceutical Ingredients (APIs) form the backbone of pharmaceutical manufacturing, and their safe, timely, and compliant movement has become increasingly critical. As a result, the demand for API logistics in the UK is growing steadily, supported by the need for robust supply chains, specialized handling, and adherence to strict quality and safety standards.
Market Overview
API logistics refers to the transportation, storage, and distribution of active pharmaceutical ingredients under controlled and regulated conditions. These logistics services are designed to ensure product integrity, regulatory compliance, and uninterrupted supply to pharmaceutical manufacturers.
In the UK, API logistics plays a vital role in supporting domestic drug production as well as international trade. APIs often require temperature-controlled environments, secure handling, and detailed documentation throughout the supply chain. Logistics providers are increasingly offering tailored solutions that address these requirements while ensuring traceability and risk mitigation.
The growing complexity of pharmaceutical products, combined with stringent regulatory oversight, has elevated API logistics from a support function to a strategic component of the pharmaceutical value chain.
Key Drivers Influencing Market Demand
One of the primary drivers fueling demand for API logistics in the UK is the increasing complexity of pharmaceutical manufacturing. Modern drugs often rely on specialized and sensitive APIs that require precise handling conditions. This has increased reliance on logistics providers with pharmaceutical-grade infrastructure and expertise.
Another significant driver is the emphasis on supply chain resilience. Disruptions in global pharmaceutical supply chains have highlighted the importance of reliable logistics networks capable of managing risks and ensuring continuity. UK-based pharmaceutical companies are increasingly prioritizing logistics partners that can provide flexibility, contingency planning, and end-to-end visibility.
Regulatory compliance also plays a major role in shaping demand. The UK pharmaceutical sector operates under strict quality and safety regulations, requiring logistics providers to adhere to Good Distribution Practices and other regulatory standards. Compliance-driven demand is pushing logistics companies to invest in certified facilities, trained personnel, and advanced monitoring systems.
Regional Insights
The demand for API logistics in the UK is concentrated around key pharmaceutical manufacturing and distribution hubs. Regions with established pharmaceutical clusters and access to ports, airports, and advanced transport infrastructure are witnessing higher demand for specialized API logistics services.
Additionally, proximity to research institutions and contract manufacturing organizations further supports regional demand. Logistics providers operating in these regions are expanding their capabilities to support both domestic distribution and international exports, reinforcing the UK’s position as a significant pharmaceutical supply chain hub.
Key Trends and Market Evolution
One of the most prominent trends in the UK API logistics market is the increasing adoption of temperature-controlled logistics solutions. APIs often require strict environmental conditions to maintain stability, leading to growing investment in cold chain infrastructure, real-time temperature monitoring, and validated transport systems.
Digitalization is another major trend shaping the market. Logistics providers are leveraging digital platforms to enhance shipment tracking, documentation management, and compliance reporting. These technologies improve transparency, reduce errors, and support regulatory audits, making them highly valuable to pharmaceutical clients.
Sustainability is also emerging as an important consideration. Companies are exploring eco-friendly packaging, optimized transport routes, and energy-efficient storage solutions to reduce the environmental impact of API logistics while maintaining compliance and safety.
Applications and End-Use Outlook
API logistics services in the UK primarily support pharmaceutical manufacturers, contract manufacturing organizations, and research institutions. These end users rely on consistent and compliant delivery of APIs to maintain production schedules and ensure product quality.
The growing outsourcing of pharmaceutical manufacturing has further increased demand for third-party logistics providers specializing in API handling. As manufacturers focus on core competencies such as drug development and formulation, logistics partners are expected to manage increasingly complex distribution requirements.
In addition, the rise of specialized and high-potency APIs is expanding the scope of logistics services. These materials often require enhanced safety protocols, secure storage, and trained handling personnel, reinforcing the need for advanced logistics capabilities.
Competitive Landscape
The UK API logistics market features a mix of global logistics providers and specialized pharmaceutical logistics companies. Competition is driven by service reliability, regulatory compliance, infrastructure quality, and technological capabilities.
Logistics companies are investing in facility upgrades, staff training, and compliance certifications to strengthen their market position. Strategic partnerships with pharmaceutical manufacturers and contract service providers are also becoming more common, enabling logistics firms to offer integrated and long-term solutions.
Innovation and service differentiation are key competitive factors, with providers focusing on value-added services such as inventory management, regulatory support, and customized transport solutions.
Conclusion
The growing demand for API logistics in the UK reflects the increasing complexity and importance of pharmaceutical supply chains. As the industry continues to evolve, reliable and compliant logistics services are essential for ensuring uninterrupted access to high-quality APIs.
For pharmaceutical manufacturers, logistics providers, and supply chain stakeholders, understanding the dynamics of the API logistics market is critical for long-term success. Access to comprehensive market insights can help organizations adapt to regulatory requirements, manage supply chain risks, and capitalize on emerging opportunities within the UK pharmaceutical ecosystem.
Browse Full Report – https://www.factmr.com/report/united-kingdom-api-logistics-market