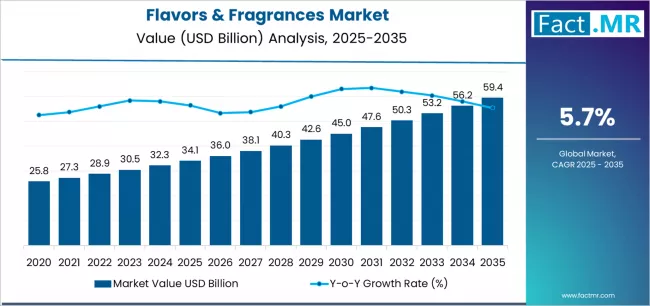
The U.S. medical-grade polypropylene (PP) market is entering a decade of steady expansion as healthcare OEMs, packagers, and converters prioritize lightweight, sterilizable, and cost-efficient polymer solutions across disposables, diagnostics, and device components. The U.S. market is estimated at roughly $1.0–1.1 billion in 2024, with projections pointing to near-doubling in value across the early-to-mid 2030s on the back of an approximate 6.5–7.1% compound annual growth rate (CAGR).
Key figures
-
U.S. medical-grade PP market size (est.): ~$1.0–1.1 billion (2024).
-
Expected U.S. growth: mid-single to high-single digit CAGR (≈6.9–7.1%) through the next decade, implying market values in the $2.0+ billion range by the early 2030s.
-
Global context: medical-grade PP demand is part of a broader materials upswing, with global medical plastics and packaging segments expanding at similar rates—supporting sustained PP uptake for medical devices and sterile packaging.
Why the market is growing
Three structural trends are driving demand for medical-grade PP in the United States:
-
Disposable and single-use device growth. Adoption of single-use syringes, fluid-management components, diagnostic cartridges, and point-of-care disposables continues to increase as healthcare providers balance infection control with cost efficiency; PP’s sterilizability, low density, and formability make it a go-to resin.
-
Medical device miniaturization and precision molding. Advances in injection molding and resin engineering are enabling thinner-walled, tighter-tolerance parts (inhaler components, diagnostic housings, connectors) where homopolymer and specially compounded PP grades excel. Manufacturers now offer medical-certified grades with documentation that accelerate device qualification.
-
Packaging and sterilized format expansion. The U.S. medical device packaging market continues to grow (sterile formats and barrier packaging), which expands demand for PP in trays, blister components, caps/closures, and primary rigid packaging—areas where PP’s clarity, stiffness, and sealing characteristics are valued.
Segment and material dynamics
Homopolymer PP is expected to remain the dominant resin type for medical applications due to its stiffness, processability, and cost profile. Random and impact copolymers will retain share where clarity, impact resistance, and toughness are required (e.g., some diagnostic housings and connectors). Across applications, medical devices and primary packaging emerge as the largest end-use channels, together accounting for a majority of U.S. demand.
Supply chain & capacity notes
U.S. PP supply is buoyed by domestic olefins and propane-dehydrogenation (PDH) capacities and strong feedstock availability, helping stabilize local prices versus import reliance for critical medical grades. Global PP producers actively market medical-certified PP grades with regulatory support and change-control processes to U.S. converters, reinforcing reliable supply for medical applications.
Innovation, regulation and sustainability
-
Regulatory readiness: Medical-grade PP manufacturers increasingly provide documentation packages and lot-traceability to speed OEM qualification—an important commercial differentiator for suppliers.
-
Circularity & recycling advances: Recyclable and low-emissions PP production footprints are becoming competitive selling points. While medical waste streams present unique sterility and contamination challenges, initiatives around mechanical and chemical recycling for non-contaminated medical PP (and design for recyclability in primary packaging) will shape mid-term supply strategies.
Risks and headwinds
Price volatility in propylene feedstock, regulatory shifts around medical waste handling, and the technical challenge of recycling contaminated streams are potential constraints. Additionally, competing high-performance polymers (some specialty polyesters and nylons) will continue to displace PP in niche high-temperature or very high-mechanical-stress applications.
Market outlook (2025–2035)
The U.S. market is expected to grow at roughly 7% CAGR through the forecast window, roughly doubling in value by the early-to-mid 2030s. Global demand expansion in medical packaging and device production—combined with ongoing resin innovation—supports this trajectory. These projections assume continued healthcare spending growth, stable sterilization practices favoring disposables, and no major sustained feedstock supply shocks.
Browse Full Report : https://www.factmr.com/report/medical-grade-polypropylene-market
Industry voices
“Medical-grade polypropylene’s balance of cost, sterilization compatibility, and processability keeps it front-and-center for both device OEMs and packagers,” said an independent polymer industry analyst. “Suppliers who bundle regulatory documentation, supply security, and recycled-content pathways will capture the lion’s share of growth.”
About this release
This market briefing presents a U.S.-focused forecast and trend analysis for medical-grade polypropylene through 2035. For data requests, bespoke forecasts, or to schedule an analyst briefing, please contact the press office below.