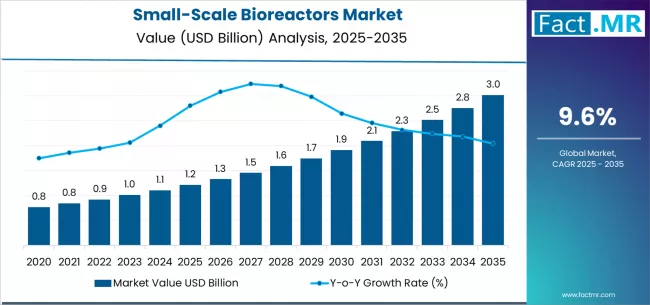
The global small-scale bioreactors market is poised for robust growth over the next decade. In 2025, the market is estimated to be worth approximately USD 1.21 billion, and by 2035 it is projected to reach around USD 3.02 billion, growing at a compound annual growth rate (CAGR) of about 9.6%. Small-scale bioreactors — typically ranging from a few milliliters up to a few liters — play a crucial role in early-stage bioprocess development, cell and microbial culture, research, and pilot-scale manufacturing. They are widely used across biopharmaceutical companies, contract research and manufacturing organizations (CROs/CMOs), and academic or research institutes.
Key Market Dynamics & Growth Drivers
-
Surge in Biopharmaceutical Pipeline & Research Activity
The expanding global biopharmaceutical industry — especially growth in biologics, monoclonal antibodies, cell and gene therapies, vaccines, and other novel therapies — is fueling demand for flexible, efficient upstream processing platforms. Small-scale bioreactors satisfy this need by enabling rapid process development, optimization, and early-stage scaling. Increasing outsourcing of R&D and manufacturing to CROs and CMOs also boosts demand, as these organizations value single-use, small-volume bioreactors for flexibility, lower contamination risk, and the ability to run multiple projects in parallel. -
Dominance of Single-Use Technology
A key trend shaping the market is adoption of single-use bioreactors, which accounted for the majority share in 2025. Compared to traditional reusable stainless-steel or glass bioreactors, single-use systems offer significant advantages: reduced risk of contamination, quicker turnaround (no cleaning/sterilization cycles), fewer validation requirements, and lower upfront infrastructure needs. These benefits have driven preference among biopharma, research labs, and contract manufacturers. -
Preferred Capacity: 1 L–3 L Leading the Market
Among capacity segments, the 1 L–3 L range is most widely used. This capacity hits a sweet spot: large enough to generate sufficient material for analytical testing or small-scale production, yet small enough to conserve media and reagents and allow efficient experimental throughput. Many bioprocess-development and R&D workflows find this scale optimal. -
Increasing Automation, Analytics & Process Control
Advances in bioreactor design — including integration of sensors, automated sampling, real-time monitoring, and control systems — are making small-scale bioreactors more powerful for process development, optimization, and scale-up readiness. These improvements enhance reproducibility, reduce variability, and align with regulatory quality-by-design expectations, encouraging broader adoption. -
Rising Outsourcing and Flexibility Needs
The growth of contract research and manufacturing, along with academic and small biotech organizations, places a premium on flexibility and rapid turnaround. Single-use small-scale bioreactors fit these requirements well because they support multi-product workflows, fast changeovers, and minimal risk of cross-contamination — a critical need when handling different cell lines, molecules, or process conditions.
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=12089
Market Segmentation Snapshot
The small-scale bioreactors market can be segmented along multiple dimensions:
• By Product: single-use systems (stirred-tank, wave-mixed, perfusion, air-lift) and reusable stainless-steel or glass bioreactors.
• By Capacity: from very small volumes (milliliters) up to around 5 L — with 1 L–3 L being the most popular.
• By End-Use: biopharmaceutical companies, CROs & CMOs, academic/research institutes, with CROs & CMOs representing a major end-use share.
• By Region: North America currently holds a significant share, owing to its established biopharma ecosystem and robust R&D infrastructure.
Challenges & Potential Restraints
Despite strong growth prospects, the small-scale bioreactors market faces some challenges:
• Cost and Supply-Chain Constraints: Single-use systems rely on disposable components. Supply chain disruptions (e.g., bag, sensor or consumable shortages) may affect availability and lead times.
• Sensor and Monitoring Limitations in Disposable Systems: Compared with reusable, heavily instrumented stainless-steel bioreactors, disposable systems may sometimes offer fewer—or less precise—analytics and process-control capabilities.
• Scaling & Transferability Challenges: Moving from small-scale (1–3 L) to larger-scale production often requires careful scale-up validation; not all bioprocess conditions translate easily, which may limit direct use of small bioreactor-derived protocols in commercial manufacturing.
• Capital Expenditure for Advanced Automation: While single-use reduces some infrastructure needs, investment in cutting-edge sensor integration, automation, and control platforms can still be non-trivial — potentially limiting adoption among smaller labs or startups.
Strategic Outlook & Future Opportunities
• Continued Single-Use Technology Innovation: As biopharma pipelines become more diverse — from monoclonal antibodies to cell and gene therapies, biologics, vaccines, and personalized medicines — demand for flexible, contamination-free, disposable bioprocessing systems will continue to rise. Single-use small-scale bioreactors will remain central to this trend, especially if manufacturers integrate advanced sensors, automation, and process-monitoring capabilities.
• Growth of Contract Services & Outsourced Bioprocessing: CROs and CMOs are likely to remain key growth engines. Their need for flexibility, multi-product throughput, and lower overhead makes single-use small reactors ideal. As outsourcing grows, demand for these systems will spread beyond traditional biopharma hubs.
• Expansion in Emerging Markets & Academic Research: Biotechnology investment in emerging economies and increased academic/research activity worldwide will drive demand for small-scale bioreactors, particularly as labs seek cost-effective, scalable, and contamination-controlled platforms.
• Integration with Digital and Quality-by-Design Frameworks: Adoption of real-time analytics, digital-twin modeling, process analytical technologies (PAT), and automation will strengthen process robustness and reproducibility, pushing small-scale reactors from “lab tools” toward integral components of bioprocess development pipelines.
Conclusion
The global small-scale bioreactors market is transforming from a niche, research-oriented toolset into foundational infrastructure for modern biopharmaceutical development. Driven by growing biologics pipelines, rising demand for flexible single-use systems, increased outsourcing to CROs/CMOs, and advances in automation and analytics, the market is expected to expand significantly from 2025 through 2035. Manufacturers and end-users who prioritize flexibility, process control, scalability, and cost-efficiency will likely lead adoption, positioning small-scale bioreactors as indispensable assets in biopharmaceutical innovation, development, and early-stage manufacturing.
Browse Full Report: https://www.factmr.com/report/small-scale-bioreactors-market