Maintaining product integrity and patient safety is a top priority in pharmaceutical manufacturing, particularly for injectable drugs packaged in ampoules. Visual inspection plays a critical role in identifying particulate contamination, cloudiness, or defects that may compromise product quality. Within this quality assurance framework, ampoule clarity test apparatus systems have emerged as essential tools for ensuring compliance with regulatory standards. As pharmaceutical production scales globally and regulatory scrutiny intensifies, the ampoule clarity test apparatus market continues to gain strategic importance across manufacturing and quality control environments.
Market Overview
Ampoule clarity test apparatus systems are designed to evaluate the visual clarity of liquid-filled ampoules by detecting suspended particles, turbidity, and other visual imperfections. These systems support manual, semi-automated, and fully automated inspection processes, depending on production scale and regulatory requirements. Their adoption is closely tied to good manufacturing practices and quality control protocols mandated by global health authorities.
The market is primarily driven by growing pharmaceutical output, increased focus on injectable drug safety, and the rising adoption of advanced inspection technologies. Manufacturers are placing greater emphasis on standardizing visual inspection methods to reduce human error while improving consistency and traceability. As a result, clarity testing apparatus solutions are becoming integral components of pharmaceutical quality assurance workflows.
Technology and Equipment Advancements
Technological innovation is shaping the evolution of ampoule clarity test apparatus systems. Traditional manual inspection methods are gradually being complemented by automated and semi-automated solutions that enhance accuracy and repeatability. Modern systems incorporate controlled lighting conditions, precision optics, and adjustable rotation mechanisms to ensure uniform inspection of ampoules.
Digital enhancements, such as image-assisted inspection and integrated monitoring interfaces, are also gaining traction. These advancements allow operators to detect minute particulate matter more efficiently while supporting documentation and audit readiness. Equipment manufacturers are focusing on ergonomic designs and user-friendly interfaces to reduce operator fatigue and improve inspection throughput in high-volume production environments.
Regulatory and Compliance Landscape
Regulatory compliance is a key factor influencing demand for ampoule clarity test apparatus solutions. Pharmaceutical manufacturers must adhere to stringent guidelines related to injectable product safety, including visual inspection requirements outlined by international regulatory bodies. Failure to meet these standards can result in product recalls, regulatory penalties, and reputational damage.
As regulatory agencies continue to emphasize contamination control and product transparency, pharmaceutical companies are investing in reliable inspection equipment to ensure consistent compliance. Ampoule clarity testing plays a vital role in meeting regulatory expectations for batch release and ongoing quality monitoring.
Regional Insights
The adoption of ampoule clarity test apparatus systems varies across regions, reflecting differences in pharmaceutical manufacturing intensity, regulatory enforcement, and technological maturity.
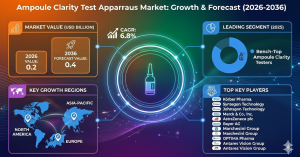
North America represents a mature market, supported by a well-established pharmaceutical industry and strong regulatory oversight. Manufacturers in this region prioritize advanced inspection technologies to meet high quality and compliance standards. Europe follows closely, driven by strict pharmaceutical regulations and widespread adoption of standardized quality control practices.
The Asia-Pacific region is emerging as a significant growth area, fueled by expanding pharmaceutical manufacturing capacity and increasing exports of injectable drugs. Countries investing in healthcare infrastructure and pharmaceutical innovation are adopting clarity testing apparatus solutions to align with global quality benchmarks and strengthen their competitive position.
Key Trends Shaping the Market
Several trends are influencing the ampoule clarity test apparatus market. One major trend is the shift toward automation in pharmaceutical inspection processes. Automated and semi-automated systems help reduce dependence on manual inspection, improving consistency while minimizing inspection-related fatigue and variability.
Another important trend is the integration of inspection equipment with broader quality management systems. Manufacturers are increasingly seeking solutions that support data capture, traceability, and compliance documentation. This integration enhances audit readiness and supports continuous improvement initiatives.
Customization and flexibility are also becoming key considerations. Equipment suppliers are offering adaptable systems that accommodate varying ampoule sizes, fill volumes, and inspection requirements, enabling manufacturers to use a single platform across multiple product lines.
Applications and End-Use Outlook
Ampoule clarity test apparatus systems are primarily used in pharmaceutical manufacturing facilities, quality control laboratories, and contract manufacturing organizations. Injectable drug producers rely on these systems to inspect ampoules during production, before labeling, and prior to market release.
Beyond traditional pharmaceutical applications, these systems are also finding use in biotechnology and specialty drug manufacturing, where product sensitivity and purity requirements are especially high. As biologics and complex injectables gain prominence, the need for precise visual inspection tools is expected to grow further.
Contract manufacturing organizations represent a particularly important end-user segment, as they must meet the quality standards of multiple clients and regulatory authorities. Reliable clarity testing equipment enables these organizations to maintain consistent quality across diverse production portfolios.
Competitive Landscape
The ampoule clarity test apparatus market features a mix of established inspection equipment manufacturers and specialized pharmaceutical technology providers. Competition is centered on reliability, inspection accuracy, regulatory compliance support, and after-sales service. Continuous product innovation and close collaboration with pharmaceutical manufacturers are essential for maintaining market relevance.
Suppliers are also focusing on global expansion strategies, including localized service support and compliance with region-specific regulatory requirements. These efforts help strengthen customer relationships and enhance long-term adoption.
Conclusion
The ampoule clarity test apparatus market plays a vital role in safeguarding injectable drug quality and ensuring regulatory compliance. As pharmaceutical manufacturing becomes increasingly complex and quality expectations rise, reliable visual inspection systems are no longer optional but essential. Continued advancements in inspection technology, combined with growing regulatory emphasis on contamination control, are expected to support sustained market development.
Access to comprehensive industry insights, such as those provided by Fact.MR, can help stakeholders understand evolving market dynamics, technology trends, and compliance requirements. For pharmaceutical manufacturers and equipment suppliers alike, investing in effective ampoule clarity testing solutions is a critical step toward ensuring product safety, regulatory confidence, and long-term operational excellence.
Browse Full Report – https://www.factmr.com/report/ampoule-clarity-test-apparatus-market