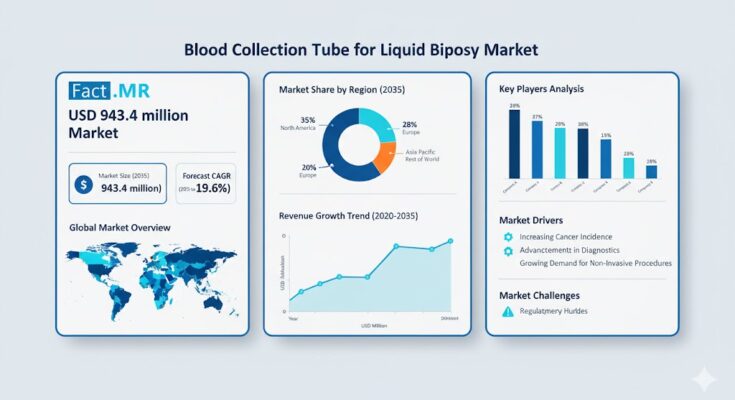
The global blood collection tube for liquid biopsy market is poised for remarkable growth, fueled by increasing adoption of non-invasive cancer diagnostic methods and advancements in genomic research. According to a recent report by Fact.MR, the market was valued at USD 157.5 million in 2025 and is forecasted to expand at a robust CAGR of 19.6%, reaching USD 943.4 million by 2035.
As healthcare systems pivot toward early disease detection, personalized therapies, and patient-friendly diagnostics, blood-based liquid biopsy solutions are redefining oncology testing and clinical decision-making.
Strategic Market Drivers
- Growing Prevalence of Cancer and Demand for Early Detection
The rising global cancer burden has intensified the need for efficient, minimally invasive diagnostic tools. Blood collection tubes designed for liquid biopsy enable the isolation and stabilization of circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA), facilitating early detection and real-time monitoring of disease progression.
Governments and healthcare providers are increasingly investing in precision diagnostics to reduce cancer-related mortality and improve treatment outcomes.
- Technological Innovations in Sample Preservation and Nucleic Acid Stabilization
Continuous advancements in tube design—such as anti-coagulation additives, cell-stabilizing agents, and enhanced polymer materials—are boosting accuracy and reliability in molecular testing. These innovations extend sample integrity, allowing laboratories to perform multi-omics analysis for clinical and research applications.
- Expanding Applications in Oncology Research and Precision Medicine
Beyond cancer diagnostics, liquid biopsy tubes are gaining prominence in genetic research, prenatal testing, and organ transplant monitoring. The shift toward personalized medicine, supported by genomics and biomarker discovery, continues to propel the adoption of specialized blood collection tubes in both hospital and research settings.
Browse Full Report: https://www.factmr.com/report/4495/blood-collection-tubes-for-liquid-biopsy-market
Regional Growth Highlights
North America: Hub of Innovation and Clinical Integration
North America leads global adoption, driven by a strong presence of biotech companies, advanced laboratory infrastructure, and supportive FDA regulatory frameworks. The U.S. remains a focal point for R&D in circulating biomarkers and next-generation sequencing (NGS)-based diagnostic assays.
Europe: Regulation and Research Synergy
Europe’s growth is supported by collaborative oncology research initiatives and government-backed healthcare programs. Germany, the U.K., and France are investing heavily in liquid biopsy technologies to enhance early cancer screening programs and reduce healthcare costs.
East Asia: Rapid Expansion and Manufacturing Excellence
East Asia, particularly China, Japan, and South Korea, is witnessing accelerated growth in diagnostic manufacturing and clinical trials. Increasing investment in biotechnology and domestic production capabilities is driving demand for advanced blood collection tubes across hospital laboratories and contract research organizations (CROs).
Emerging Economies: Rising Awareness and Healthcare Access
Regions such as Latin America, the Middle East, and South Asia are showing growing potential as awareness of early cancer detection expands. Improving healthcare infrastructure and rising participation in global clinical trials are expected to further boost adoption in these regions.
Challenges and Market Considerations
Despite exponential growth, several factors influence market dynamics:
- High Cost of Liquid Biopsy Procedures: Limits widespread adoption in developing economies.
- Regulatory Complexity: Strict compliance requirements for in vitro diagnostic (IVD) devices may delay approvals.
- Technical Sensitivity: Maintaining nucleic acid integrity during transport and storage remains a key challenge.
- Competition from Conventional Biopsy and Emerging Sampling Methods: Alternatives may constrain rapid transition.
Competitive Landscape
The global market is characterized by innovation-led competition and strategic partnerships between diagnostics firms and research institutes.
Prominent players include:
- Biocept Inc.
- F. Hoffmann-La Roche Ltd
- Streck Inc.
- Norgen Biotek Corp
- Exact Sciences Corp
- MagBio Genomics Inc
- Zymo Research Corporation
- Apostle Sciences
- QIAGEN NV
- Greiner Bio-One International GmbH
These companies are focusing on next-generation tube technologies, workflow automation, and clinical validation to strengthen their market presence.
Recent Developments
- 2023: Streck Inc. launched new cfDNA BCT tubes optimized for extended sample preservation and high-yield plasma recovery.
- 2022: QIAGEN NV introduced PAXgene Blood ccfDNA Tubes designed for precise pre-analytical handling in liquid biopsy assays.
- 2022: Greiner Bio-One expanded its VACUETTE® range with improved stabilization agents for CTC isolation.
Future Outlook: Shaping the Next Era of Non-Invasive Diagnostics
The next decade will mark a paradigm shift in oncology diagnostics as liquid biopsy technologies become integral to early cancer detection and treatment monitoring. With rapid advancements in bioinformatics, AI-driven data interpretation, and sample stabilization chemistry, the blood collection tube for liquid biopsy market is set to witness sustained innovation and global adoption.
Companies that prioritize collaboration, standardization, and patient-centric innovation will define the future of this dynamic industry—transforming how cancer and other complex diseases are diagnosed and managed worldwide.