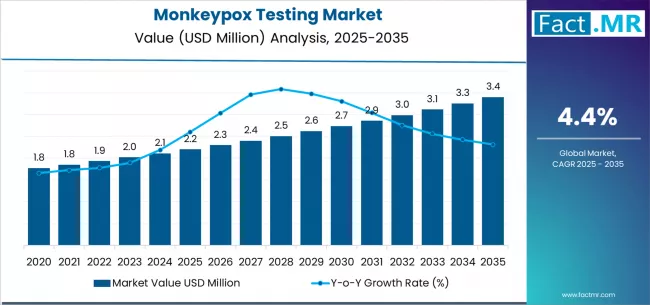
The global market for monkeypox testing has emerged as a critical component of infectious-disease diagnostics, especially in light of recent outbreaks and renewed global attention on zoonotic viruses. As the disease spreads into newer geographies and public-health authorities emphasize early detection and containment, demand for reliable, rapid, and accessible monkeypox diagnostic solutions is growing steadily. With rising awareness, improved surveillance networks, and investment in diagnostic infrastructure, the market is poised for a significant expansion over the next decade.
In 2024, the market was already registering substantial revenues globally, and forecasts suggest steady growth through 2030 and beyond, reflecting increasing incidence of monkeypox infections, expanded screening programs, and rising demand for diverse testing technologies.
Market Segmentation & Key Technologies
Monkeypox testing currently incorporates several methods, catering to different testing needs — from high-sensitivity laboratory diagnostics to rapid point-of-care screening. Key segments include:
-
Molecular Diagnostics (PCR-based tests): Polymerase Chain Reaction (PCR) remains the backbone of monkeypox detection, widely preferred for its accuracy and reliability.
-
Rapid Antigen / Point-of-Care Tests: Rapid diagnostic kits and lateral flow assays (LFA) are gaining traction, especially in resource-constrained or outbreak-response settings where quick results matter.
-
Serological / Immunoassay Tests and Other Technologies: For broader surveillance and antibody detection, serological tests and advanced immunoassays are used.
-
Next-Generation & Emerging Diagnostics: Innovations such as genetic sequencing, biosensor-based assays, and multiplex platforms are becoming more relevant — especially for future-proofing diagnostics and enabling scalable surveillance.
As for end-users, testing is conducted in:
-
Hospitals and clinical settings
-
Diagnostic laboratories and public-health labs
-
Research and academic institutions
-
Mobile/field-deployable testing units (in outbreak zones or low-resource regions)
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=12008
Growth Drivers Fueling Market Expansion
1. Rising Incidence & Recurring Outbreaks
Resurgent monkeypox cases globally — including in regions previously non-endemic — remain the primary driver of testing demand. Increased human-to-human transmission, global travel, and changing patterns of zoonotic spillover are prompting governments and health agencies to ramp up testing capacities, thereby boosting market growth.
2. Heightened Surveillance and Public-Health Initiatives
Following outbreaks, many countries are strengthening disease monitoring, surveillance, and containment efforts. This involves integrating monkeypox diagnostics with broader public-health response frameworks, increasing demand for high-throughput testing, rapid diagnostics, and scalable assay kits.
3. Technological Advancements in Diagnostics
Advances in molecular diagnostics, portable PCR platforms, point-of-care antigen/antibody kits, and emerging sequencing/biosensor technologies are improving accuracy, speed, and accessibility. These innovations reduce turnaround time, lower the requirement for advanced lab infrastructure, and enable testing in decentralized or resource-limited settings.
4. Demand for Rapid, Accessible, Point-of-Care Testing
In outbreak scenarios and low-resource regions, there is a strong push for rapid tests that deliver quick results without requiring sophisticated laboratory infrastructure. Lateral flow assays and immunoassays offer the advantage of ease of use, minimal training requirements, and quick turnaround — making them vital for responsive public-health efforts.
5. Strengthening Global Diagnostic Infrastructure & Funding
International funding, global health agency support, and investments in diagnostic supply chains are enhancing testing infrastructure. Improved logistics, better diagnostic kit availability, and expansion of lab networks are allowing widespread deployment of monkeypox testing capabilities.
Challenges & Market Constraints
Despite the positive outlook, several challenges could impede market growth:
-
Limited Testing Infrastructure in Low-Resource Regions: Many developing countries still lack adequate labs, trained personnel, or cold-chain logistics — limiting access to high-quality testing.
-
High Cost & Supply-Chain Bottlenecks: Advanced molecular and antigen kits can be expensive, and disruptions in raw material supply or logistics can hamper kit availability and timely distribution.
-
Regulatory Hurdles & Standardization Issues: Because monkeypox testing protocols vary across countries, regulatory approvals, validation procedures, and quality standards may delay kit deployment, particularly for novel or rapid tests.
-
Public Awareness, Stigma & Testing Hesitancy: In some regions, lack of awareness, misinformation, or stigma associated with the disease can lead to under-testing, which undermines disease surveillance and management efforts.
Strategic Outlook & Opportunities
For Diagnostic Manufacturers & Kit Developers
-
Innovate affordable, high-sensitivity rapid test kits — such as point-of-care antigen or immunoassay kits — that are easy to deploy in remote or resource-limited settings.
-
Expand capacity for PCR-based and multiplex testing solutions to meet high-throughput demand during outbreak peaks.
-
Collaborate with governments and global health agencies to supply kits for national screening programs and outbreak response.
For Healthcare Providers & Public-Health Agencies
-
Integrate monkeypox testing into routine diagnostic panels — especially in regions with recurrent outbreaks or heightened zoonotic risk.
-
Deploy mobile testing units and community-level rapid testing campaigns to ensure broader coverage, especially in underserved areas.
-
Invest in training laboratory personnel and ensuring quality-control protocols for reliable diagnostic outcomes.
For Investors & Policy Makers
-
Support research into next-generation diagnostics (biosensors, sequencing, AI-driven detection) to future-proof testing infrastructure.
-
Fund public-health infrastructure upgrades and subsidize testing in low-income or high-risk regions to enhance outbreak response and disease surveillance.
-
Encourage public awareness campaigns to reduce stigma, increase testing uptake, and improve early detection rates.
Market Forecast & Long-Term Outlook
Looking ahead over the next 5–10 years, the monkeypox testing market is expected to grow steadily, supported by recurring outbreaks, global surveillance efforts, and continuous diagnostic innovation. Estimates from recent analyses suggest that market valuation may reach into the billions of USD globally by the early-to-mid 2030s (depending on outbreak frequency, public-health investments, and adoption of new diagnostic technologies).
With continued expansion in point-of-care diagnostics, decentralized testing capabilities, and integration with broader infectious-disease surveillance frameworks, monkeypox testing is likely to remain a key pillar of global public health preparedness. Manufacturers, health agencies, and governments that prioritize accessibility, affordability, and diagnostic accuracy stand to play a central role in shaping the future of this market.
Browse Full Report: https://www.factmr.com/report/monkeypox-testing-market