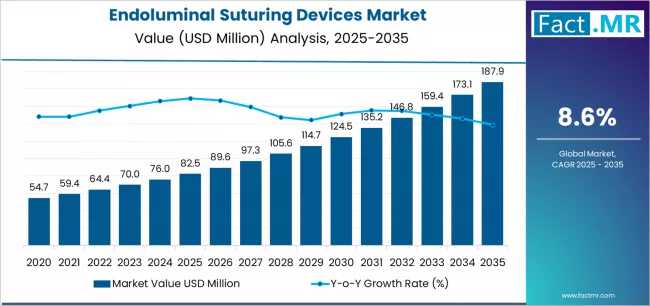
The global endoluminal suturing devices market is projected to expand significantly over the next five years, reaching $1.2 billion by 2030, growing at a CAGR of 9.3% between 2025 and 2030, according to the latest market intelligence. This growth is fueled by increasing prevalence of gastrointestinal disorders, rising bariatric procedures, and rapid advancements in device technology.
Endoluminal suturing devices, which allow tissue approximation through natural orifices, are revolutionizing gastroenterology and bariatric surgeries. These devices reduce patient recovery time by up to 50% compared to conventional surgery and have reported clinical success rates exceeding 90% in tissue repair and endoscopic sleeve gastroplasty procedures.
Market Drivers
-
Rising Gastrointestinal Disorders: Globally, GERD affects 20% of adults in Western countries, while peptic ulcer disease impacts 10% of the population, increasing demand for minimally invasive interventions.
-
Obesity Epidemic: According to WHO data, over 650 million adults are classified as obese worldwide, driving adoption of endoscopic bariatric suturing procedures, which have grown by 12% annually in North America over the past three years.
-
Technological Advancements: Robotic-assisted suturing systems and single-use disposable devices have improved procedural precision, with robotic systems reducing suturing errors by over 30% compared to manual techniques.
Regional Market Insights
-
North America: Holds 45% of the global market, driven by advanced hospital infrastructure, high procedure volumes, and reimbursement coverage. The U.S. alone performed over 12,000 endoluminal suturing procedures in 2024.
-
Europe: Accounts for 30% of the market, led by Germany, France, and the UK. Growth is supported by government initiatives and favorable reimbursement policies.
-
Asia-Pacific: Projected to grow at a CAGR of 11.2%, with Japan, China, and India leading adoption due to rising healthcare expenditure and expanding surgical centers.
Market Segmentation
-
By Product Type: Manual suturing devices, automated suturing systems, robotic-assisted devices. Automated and robotic-assisted systems are expected to grow at a CAGR of 10.5%, reflecting surgeon preference for precision and reduced learning curves.
-
By Procedure Type: Gastrointestinal repair dominates with 55% of procedures globally, while bariatric applications are the fastest-growing segment, expanding at 13% CAGR due to increasing obesity rates.
-
By End-Use: Hospitals remain the primary end-user, capturing 70% of market share, followed by ambulatory surgical centers.
Competitive Landscape
The market is highly competitive. Key players include Apollo Endosurgery, Medtronic, Ethicon (Johnson & Johnson), CONMED Corporation, and Boston Scientific. Strategic initiatives such as AI-integrated suturing platforms and clinician training programs are increasing adoption rates. For example, Apollo Endosurgery’s OverStitch platform has achieved over 30,000 procedures globally since launch, demonstrating clinical acceptance and market traction.
Challenges and Opportunities
While growth is robust, challenges include high device costs, limited trained surgeons, and regulatory hurdles in certain regions. Nevertheless, the market opportunity is significant: analysts estimate that emerging markets in Asia-Pacific could account for $150 million in revenue by 2030, driven by cost-effective device innovation and expanding hospital infrastructure.
Conclusion
The global endoluminal suturing devices market is poised for strong expansion, driven by technological innovation, rising minimally invasive surgeries, and increasing gastrointestinal and obesity-related procedures. Companies focusing on device innovation, AI integration, and strategic hospital partnerships are well-positioned to capitalize on the projected growth.
Browse Full Report : https://www.factmr.com/report/endoluminal-suturing-devices-market