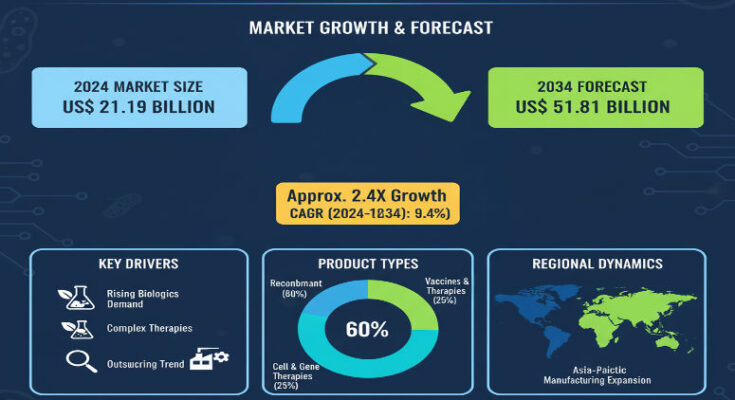
In 2024, the biologics contract manufacturing market is estimated at about USD 21.19 billion. Over the ten-year window through 2034, the market is forecast to grow at a compound annual growth rate (CAGR) of approximately 9.4%, reaching a projected value of about USD 51.81 billion by the end of 2034. Key segments showing especially strong predictions include oncology biologics, mammalian expression platforms, and commercial manufacturing applications.
Market Drivers & Trends
Several converging forces are powering this growth. First, rising prevalence of chronic diseases and the ongoing growth of personalized medicine, including biologics, have increased pressure on existing manufacturing capacity. Second, constructing and maintaining fully compliant biologics manufacturing facilities (with clean rooms, high purity requirements, etc.) demands large investments and long lead times. Outsourcing to established CMOs offers faster scale-up and mitigates risk. Third, advances in biotechnology—better cell culture systems, genetic engineering, single-use technologies, automation—are increasing both the complexity and the value of biologic therapeutics, pushing companies to rely on partners with specialized expertise. Finally, regulatory complexity and quality assurance are increasingly important; companies seek CMOs with robust track records in compliance, quality, and scale.
Recent Developments & Innovation
Recent years have witnessed several important developments in the biologics contract manufacturing landscape. Many CMOs are investing in new capacity, especially for mammalian cell culture systems, to meet demand for monoclonal antibodies and recombinant proteins. There is also a growing trend of mergers, acquisitions, and strategic partnerships, as firms aim to offer more integrated end-to-end biologics CDMO services spanning R&D, clinical manufacturing, and commercial scale-up. Advances in single-use bioreactors, process automation, analytics, and digital manufacturing are helping reduce costs, shorten lead times, and improve reproducibility. Oncology remains a high growth area; contract manufacturing for oncology biologics is expected to reach substantial revenues by 2034 as more therapies enter late development. Emerging markets, especially in East Asia, are seeing accelerated facility investments and greater local CMO activity to serve regional demand.
Key Players & Competitive Landscape
Prominent names in the biologics contract manufacturing market include Lonza Group AG, Samsung Biologics, AGC Biologics, Fujifilm Diosynth Biotechnologies, BioXcellence, Patheon, Ajinomoto Bio-Pharma, Emergent BioSolutions, Merck KGaA, Rentschler Biotechnologies, Avid Bioservices, Novasep, KBI Biopharma, Therapure Biopharma, and Abzena. These companies compete on multiple fronts: platform technology (mammalian versus microbial expression), scale of manufacturing (clinical vs commercial scale), regulatory and quality reputation, speed to clinic, geographical footprint, and cost. Those CMOs that can combine high-quality regulatory compliance, flexible capacity, and innovative platforms (e.g. for newer biologic constructs or complex modalities) are positioned best for leadership.
Regional Insights: United States & Asia-Pacific
United States
The U.S. market remains a central force. A strong biotech ecosystem, substantial R&D funding, favorable regulatory pathways, and demand for oncology and advanced biologics bolster outsourcing. U.S. CMOs are expanding capacity and service offerings, while biotech firms increasingly prefer to partner with CDMOs rather than invest fully in in-house manufacturing. Growth rate for the U.S. is projected to mirror or slightly exceed global CAGR, thanks to high demand and scale.
Asia-Pacific (notably East Asia including China)
Asia-Pacific is one of the fastest-growing regions. China, in particular, is projected to grow at double-digit CAGR, with increasing internal demand coupled with global outsourcing interest. Many multinational drug companies are expanding operations or supply chains into Asia, attracted by cost efficiencies, existing biopharma infrastructure growth, and supportive government policies. Local CMOs are investing heavily in mammalian production platforms to serve both domestic and international demand.
Segment Insights: Product Types, Platforms & Applications
Among product types, monoclonal antibodies remain a dominant category. By 2034, they are expected to account for approximately 38% of market value, driven by their wide therapeutic use, high cost per unit, and the complexity of their manufacturing. The mammalian expression platform is projected to hold a large majority of the platform-based market share (over 70%), owing to their use for many high-value biologics. In contrast, microbial platforms serve less complex proteins or earlier stage modalities. On the application front, the commercial segment (versus clinical development) is set to command a substantial share of revenue (~74%) by 2034, as approved biologics scale up, and CMOs fill demand for large volume production. Among therapeutic areas, oncology is leading, followed by autoimmune, infectious diseases, and metabolic disorders.
Challenges & Barriers
While the forecast is strong, several challenges could restrain growth. The high cost of facilities compliant with biologic GMP standards, rigorous regulatory requirements, and long lead times are significant entry barriers, especially in emerging markets. Talent shortages—specialist bioprocess engineers, quality assurance experts—are also a concern. Supply chain weaknesses, especially for raw biologic materials, single-use consumables, and specialized components, could pose risks. Clinical and late-stage failure of biologic drug candidates also impact demand forecasting for CMOs. Additionally, pressure for cost reductions and price competition may compress margins in commoditized biologics.
Browse Full Report: https://www.factmr.com/report/biologics-contract-manufacturing-market
Outlook & Strategic Recommendations
Looking ahead to 2034, the biologics contract manufacturing market is expected to more than double in value. Strategic success will favor CMOs and CDMOs that can deliver scale, flexibility, regulatory excellence, technological innovation, and regional presence. Companies that invest in high-throughput mammalian platforms, single-use systems, digital manufacturing, and integrated services from clinical to commercial will be best positioned. For biotech firms, partnering with CMOs that can rapidly scale and ensure quality can significantly de-risk product development. Governments and regulators will have important roles in streamlining approval pathways, encouraging capacity building, and ensuring quality infrastructure in emerging markets.