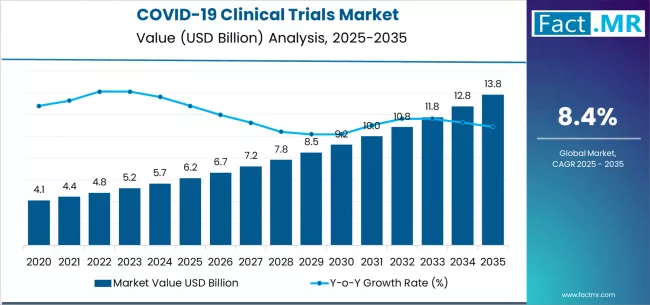
The global COVID-19 clinical trials market is on a trajectory of strong long-term expansion, driven by continued R&D investments, evolving virus variants, advanced vaccine technologies, and strengthened global preparedness for future pandemics. According to a recent analysis, the market is projected to grow from USD 6.15 billion in 2025 to USD 13.83 billion by 2035, recording an absolute increase of USD 7.68 billion and expanding at a CAGR of 8.4% during the forecast period.
As the world shifts from emergency pandemic response to strategic, long-term infectious disease management, COVID-19 clinical trials remain a critical pillar supporting new therapeutics, booster vaccines, diagnostics, and combination treatment research.
Key Market Drivers
- Rising Need for Variant-Targeted Therapeutics and Vaccines
The virus continues to evolve, prompting pharmaceutical companies and research institutions to develop next-generation solutions. Clinical trials are increasingly focused on variant-resistant vaccines, combination antivirals, monoclonal antibodies, and long-term immunity boosters. This sustained research momentum is a key driver of market growth.
- Expansion of Global Pandemic Preparedness Programs
Governments and global organizations are investing heavily in pandemic-readiness frameworks. Programs supporting advanced clinical research infrastructures, decentralized trial models, and increased funding for infectious disease R&D are enhancing trial activity across regions.
- Accelerated Adoption of Decentralized & Digital Clinical Trial Models
The urgent timelines during the pandemic accelerated adoption of remote monitoring, digital patient data collection, AI-driven analytics, and hybrid clinical trial designs. These efficiencies continue to shape the post-pandemic research landscape, enabling faster, safer, and more cost-effective trials.
- High Demand for Post-Infection and Long COVID Therapies
Persistent cases of long COVID and post-infection complications are boosting clinical trial investment in respiratory, cardiovascular, neurological, and immune-related treatment solutions. This expanding therapeutic pipeline contributes significantly to market growth.
Browse Full Report: https://www.factmr.com/report/covid-19-clinical-trials-market
Regional Growth Highlights
North America: Largest Market with Strong R&D Infrastructure
The U.S. continues to dominate global clinical trial activity due to its advanced biotechnology sector, strong regulatory ecosystem, and extensive research funding. Collaborations between pharmaceutical companies, CROs, and academic institutions further accelerate trial initiation and completion.
Europe: Leadership in Collaborative Research & Regulatory Support
Europe’s integrated research networks and strong emphasis on vaccine development, immunotherapies, and public health monitoring support robust market growth. Regulatory flexibility and cross-border clinical research coordination also contribute to rising trial volumes.
Asia-Pacific: Fastest-Growing Region
Countries such as China, India, South Korea, and Australia are rapidly expanding clinical research infrastructure. Cost-effective trial capabilities, rising CRO presence, supportive government initiatives, and large patient pools make Asia-Pacific a high-potential region for future expansion.
Latin America & Middle East/Africa: Emerging Markets with Growing Opportunities
Increasing participation in global trials, expanding healthcare infrastructure, and rising epidemiological surveillance capabilities are improving trial visibility and investment flow into these regions.
Market Segmentation Insights
By Study Type
- Interventional Studies – Remain dominant due to high demand for vaccine and therapeutic efficacy testing.
- Observational Studies – Growing focus on real-world evidence, long-term safety, and post-infection impacts.
- Expanded Access Trials – Strong relevance for high-risk patient populations needing investigational therapies.
By Phase
- Phase I & II Trials – Driven by next-generation vaccine pipelines and novel therapeutic candidates.
- Phase III Trials – Significant share due to large-scale efficacy studies.
- Phase IV Studies – Increasing importance for post-marketing surveillance and real-world safety monitoring.
By Sponsor
- Pharmaceutical & Biotech Companies – Lead investments in antiviral drugs, vaccine innovation, and biologics.
- Academic & Research Institutions – Critical contributors to diagnostics and epidemiological research.
- Government & Non-profit Organizations – Support cooperative research initiatives and global health missions.
Market Challenges
Despite strong growth prospects, the COVID-19 clinical trials market faces notable constraints:
- High R&D and Operational Costs: Complex protocols and multi-country trial requirements increase costs.
- Regulatory Variability Across Regions: Differing timelines and approval frameworks can delay progress.
- Patient Recruitment Challenges: Declining case numbers in some regions may affect trial enrollment.
- Competition with Broader Infectious Disease Research: Funding distribution across multiple pathogens may impact COVID-19-specific programs.
Competitive Landscape
The market features a mix of pharmaceutical giants, biotechnology innovators, and contract research organizations (CROs) pursuing accelerated R&D. Companies are prioritizing:
- Rapid vaccine updates targeting emerging variants
- Novel antiviral and monoclonal antibody development
- Digital and decentralized clinical trial models
- Global partnerships with government and academic organizations
Prominent players include:
- Pfizer Inc.
- Moderna Inc.
- AstraZeneca
- Johnson & Johnson
- Roche
- GSK
- Novartis AG
- ICON plc
- IQVIA
- Labcorp Drug Development
These companies continue to drive innovation, regulatory approvals, and global trial expansion.
Future Outlook: Toward a New Era of Pandemic-Ready Clinical Research
The next decade will redefine infectious disease research as COVID-19 transitions from a crisis to a long-term public health priority. The market will witness:
- Increased investment in universal coronavirus vaccines
- Growth of AI-enabled predictive clinical trial models
- Expansion of real-world evidence programs
- Greater focus on long COVID therapeutic development
- Strengthened international collaborations for global trial harmonization
Clinical trial sponsors that embrace digital transformation, invest in variant-targeted therapies, and build resilient global research networks stand to gain significant advantages as the market accelerates toward 2035.