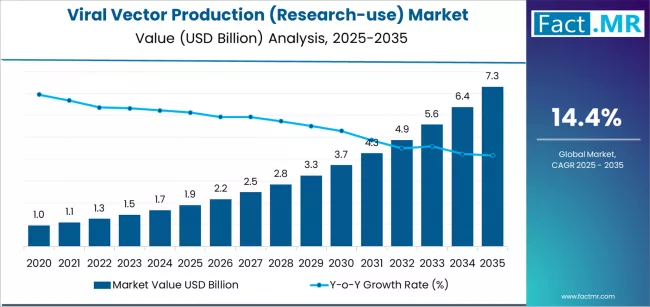
The global viral vector production (research-use) market is set for exponential growth over the next decade, driven by rising investments in gene therapy R&D, significant advancements in biomanufacturing technologies, and expanding utilization of viral vectors in academic and preclinical studies. According to a new analysis by Fact.MR, the market is projected to grow from USD 1.9 billion in 2025 to USD 7.3 billion by 2035, marking an absolute increase of USD 5.4 billion. This reflects a robust CAGR of 14.4%, translating into an overall growth of 284.2% during the assessment period.
As gene-editing platforms, cell therapy pipelines, and molecular biology applications expand globally, demand for high-quality, research-grade viral vectors is surging. The need for safer, scalable, and more efficient delivery systems is further fueling the uptake of various vector types, including AAV, lentiviral, retroviral, and adenoviral vectors.
Strategic Market Drivers
Gene Therapy and Cell Therapy R&D Accelerate Demand
The rapid advancement of gene therapy technologies and the growth of cell-based therapeutics are propelling the need for viral vectors as essential delivery tools. Research organizations, biotech startups, and academic institutions increasingly rely on vectors for CRISPR-based editing, functional genomics, and translational studies. The expanding pipeline of next-generation therapies is creating strong, consistent demand for scalable vector production.
Biomanufacturing Innovations Improve Yield & Quality
Breakthroughs in upstream and downstream bioprocessing—including suspension cell cultures, single-use systems, and chromatography improvements—are elevating vector yield, purity, and reproducibility. Automation, AI-driven optimization, and closed-system workflows are enabling manufacturers to reduce contamination risks and shorten development timelines.
Rising Academic Collaborations Strengthen Market Expansion
Universities, research institutes, and government-funded laboratories are boosting investments in genetic research. Demand is further amplified by collaborative projects, shared manufacturing facilities, and rising grant allocations for advanced gene modification studies.
Browse Full Report: https://www.factmr.com/report/viral-vector-production-research-use-market
Regional Growth Highlights
North America: The Global Innovation Hub
North America dominates the market with a strong presence of leading biotech and research organizations. Substantial NIH funding, thriving gene therapy pipelines, and the presence of advanced contract development and manufacturing organizations (CDMOs) are accelerating regional growth.
Europe: Strong Academic Research and Regulatory Support
Europe’s emphasis on high-quality biomanufacturing, along with supportive regulatory frameworks, is boosting demand for viral vectors. Countries such as Germany, the U.K., Switzerland, and France are expanding research infrastructures and investing in oncology, rare diseases, and regenerative medicine.
East Asia: Rapidly Growing Biotech Ecosystem
China, Japan, and South Korea are emerging as major players due to rapid advancements in biotechnology, government funding for genomics, and expansion of local CDMOs. The growing academic adoption of gene-editing tools is further accelerating market expansion.
Emerging Markets: Expanding Research Capabilities
Countries in Latin America, India, and Southeast Asia are experiencing rising investments in life sciences research, supported by strengthening academic networks and increasing participation in global clinical development programs.
Market Segmentation Insights
By Vector Type
- AAV Vectors – Dominant due to high safety and effectiveness in gene therapy research
- Lentiviral Vectors – Widely used in CAR-T and stem cell research
- Adenoviral Vectors – Preferred for high transduction efficiency
- Retroviral Vectors – Utilized for stable gene integration
By Application
- Gene Therapy Research
- Cell Therapy Research
- Preclinical Studies
- Genetic Engineering & Functional Genomics
By End User
- Academic & Research Institutes – Largest consumers of research-grade vectors
- Biotechnology Companies – Increasing use for early-stage therapeutic development
- Contract Research Organizations (CROs) – Fast-growing segment due to outsourcing trends
Challenges Impacting Market Growth
- Complex Manufacturing Processes – High expertise and advanced infrastructure required
- High Production Costs – Complex workflows and quality control increase expenses
- Biosafety & Regulatory Constraints – Stringent standards for vector purity and safety
- Scalability Limitations – Difficulties in transitioning from lab-scale to larger batches
Competitive Landscape
The viral vector production (research-use) market is moderately concentrated, with players focusing on enhancing manufacturing capabilities, improving vector potency, and expanding R&D collaborations.
Key Companies Profiled:
- Thermo Fisher Scientific
- Merck KGaA
- Takara Bio
- Lonza Group
- Charles River Laboratories
- Catalent
- Wuxi AppTec
- Oxford Biomedica
- Aldevron
- Vigene Biosciences
Manufacturers are increasingly adopting next-gen technologies such as AI-driven process optimization, single-use bioreactors, and transient transfection systems to improve scalability and efficiency.
Recent Developments
- 2024: Expansion of contract viral vector manufacturing services focusing on AAV and lentiviral vectors
- 2023: Launch of high-yield vector production platforms for academic and preclinical research
- 2022: Strategic partnerships between biotech startups and CDMOs to accelerate vector development
Future Outlook: A Decade of Breakthrough Genetic Research
The coming decade will be marked by massive growth in genetic engineering, gene editing, and cell therapy research—areas where viral vectors remain indispensable. Investments in scalable, high-quality vector manufacturing, combined with rising academic research output, will drive sustained market expansion.
With strong adoption across research institutions and biotechnology innovators, the global viral vector production (research-use) market is well-positioned for transformative growth through 2035.