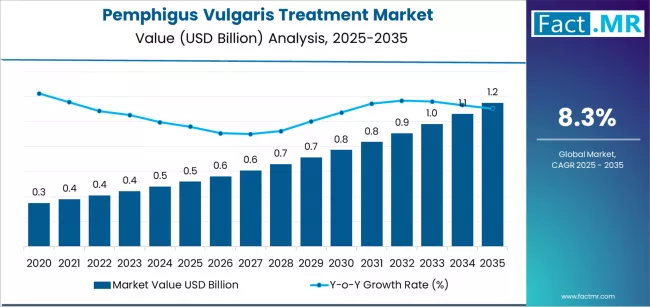
The global pemphigus vulgaris treatment market is poised for significant expansion over the next decade, driven by rising awareness, advancements in immunotherapy, and increasing access to specialized dermatological care. As per a recent analysis by Fact.MR, the market is projected to grow from USD 0.52 billion in 2025 to USD 1.15 billion by 2035, marking an absolute increase of USD 0.63 billion and a strong CAGR of 8.3% over the forecast period.
Pemphigus vulgaris—an autoimmune blistering disorder requiring long-term medical intervention—has witnessed major therapeutic improvements with the introduction of biologics, steroid-sparing agents, and targeted therapies. The growing number of clinical trials and improved disease management protocols are further boosting demand.
Strategic Market Drivers
Rising Early Diagnosis and Improved Disease Awareness
Greater awareness among healthcare professionals, improved diagnostic criteria, and increased patient education are allowing for quicker identification of pemphigus vulgaris. Early diagnosis minimizes complications, reduces hospitalization costs, and increases uptake of advanced therapies, driving the demand for corticosteroids, immunosuppressants, and biologics.
Breakthrough Biologics Transforming Treatment Outcomes
Biologics such as rituximab have become first-line therapy due to their ability to reduce relapse rates and minimize long-term steroid use. Ongoing R&D in B-cell–targeted therapies, monoclonal antibodies, and novel immunomodulators is reshaping the treatment landscape and creating opportunities for pharmaceutical innovators.
Strengthening Healthcare Access & Integration of Specialty Care
Growing investments in dermatology centers, expanded autoimmune disease clinics, and improved reimbursement frameworks are ensuring wider access to pemphigus vulgaris treatments. Teledermatology and digital patient monitoring are emerging as supportive tools to enhance treatment adherence and follow-up care.
Innovation in Treatment Protocols Elevates Patient Outcomes
Advanced protocols combining corticosteroids with biologics are reducing adverse effects and improving remission rates. Research-backed regimens, personalized treatment pathways, and real-world clinical evidence are enabling better long-term management of this chronic condition.
Browse Full Report: https://www.factmr.com/report/2185/pemphigus-vulgaris-treatment-market
Regional Growth Highlights
North America: Dominating with Biological Advancements
The U.S. continues to lead due to significant investment in clinical research, widespread adoption of biologics, and strong presence of specialty care providers. Favorable insurance coverage is further propelling market growth.
Europe: Strong Clinical Trial Activity and Regulatory Support
Countries such as Germany, France, and the U.K. are advancing with high patient awareness and robust clinical studies, alongside strong regulatory backing for biologic therapies.
East Asia: Rising Healthcare Infrastructure and Diagnosis Rates
Japan, China, and South Korea are witnessing increased pemphigus vulgaris diagnosis due to improved healthcare access and dermatological expertise. Investments in advanced biologics are strengthening regional demand.
Emerging Markets: Expanding Access and Affordability
India, Brazil, and Southeast Asian countries are experiencing increased uptake of immunosuppressants and affordable biosimilar options, supported by expanding healthcare systems.
Market Segmentation Insights
By Drug Class
- Corticosteroids – Widely used as first-line therapy.
- Immunosuppressants – Growing demand as supportive therapy.
- Biologics (Rituximab, Others) – Fastest-growing segment due to superior efficacy.
- IVIG (Intravenous Immunoglobulin) – Effective for refractory cases.
By Route of Administration
- Oral – Dominant for long-term management.
- Injectable/IV – Rising usage for biologics and IVIG therapies.
By Distribution Channel
- Hospital Pharmacies – Major distribution due to disease severity.
- Specialty Clinics & Dermatology Centers – Strong patient preference.
- Online Pharmacies – Growing adoption for chronic therapy refills.
Key Challenges
- High Cost of Biologic Treatments – Limits adoption in middle- and low-income regions.
- Side Effects of Long-Term Steroid Use – Drives need for safer alternatives.
- Limited Awareness in Developing Regions – Delayed diagnosis impacts outcomes.
- Supply Chain Dependencies for Advanced Drugs – Affect continuity of care.
Competitive Landscape
The market is moderately competitive, with companies emphasizing clinical research, biosimilar development, and improved treatment access.
Key Companies Profiled
- Roche
- Sanofi
- Pfizer
- Novartis
- GlaxoSmithKline (GSK)
- Teva Pharmaceutical Industries
- Dr. Reddy’s Laboratories
- Sun Pharmaceutical Industries
- CSL Behring
- Baxter International
Innovators are focusing on next-generation monoclonal antibodies, reduced-toxicity regimens, and enhanced patient support programs.
Recent Developments
- 2024: New biologic combinations evaluated for improved remission in moderate-to-severe pemphigus vulgaris.
- 2023: Expanded approval of rituximab-based therapies across multiple global markets.
- 2022: Introduction of personalized treatment algorithms and digital monitoring technologies to improve patient adherence.
Future Outlook: A Decade of Advanced Autoimmune Therapies
The next decade will be shaped by biologics, biosimilars, and personalized medicine that offer safer, more effective disease control. While demand for corticosteroids and immunosuppressants will persist, biologics will continue to define therapeutic progress.
With rising diagnosis, improving treatment access, and continuous pharmaceutical innovation, the pemphigus vulgaris treatment market is set for robust and sustained growth through 2035.