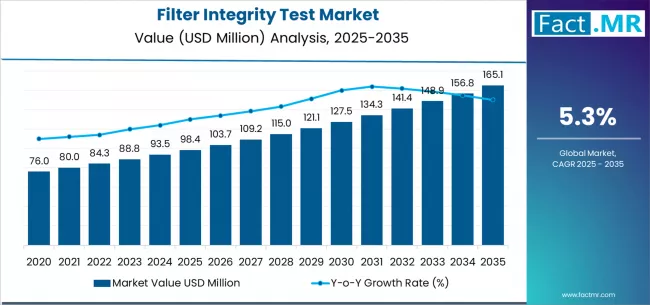
The global filter integrity test market is on a steady upward trajectory, supported by the increasing need for reliable sterilization filtration, stringent regulatory requirements, and rapid expansion of biopharmaceutical and food-processing industries. According to a recent analysis by Fact.MR the market is projected to grow from USD 98.43 million in 2025 to USD 165.11 million by 2035, reflecting a notable CAGR of 5.3% and an absolute growth of USD 66.68 million during the forecast period.
Filter integrity testing—essential for confirming the performance of sterilizing-grade filters—has become integral in pharmaceutical manufacturing, biologics processing, water purification, food safety protocols, and industrial quality control.
Key Market Drivers
Escalating Regulatory Pressure on Sterile Filtration
Global regulatory authorities, including the FDA and EMA, mandate rigorous validation and verification of sterilizing filters used in pharmaceutical and biotechnology processes. These growing compliance requirements are fueling demand for advanced filter integrity test systems that ensure precision, consistency, and audit-ready traceability.
Manufacturers increasingly rely on automated testing systems to minimize human error and maintain uninterrupted production in aseptic environments.
Expansion of Biopharmaceutical Manufacturing
The rise in biologics, vaccines, cell therapies, and monoclonal antibodies has significantly increased the need for sterile filtration to maintain product purity. Filter integrity testers—such as bubble point, pressure decay, and diffusion flow systems—play a critical role in preventing contamination and ensuring the safety of high-value biologic products.
This trend directly boosts investments in reliable, high-performance filtration validation equipment.
Growing Importance of Water and Food Safety
The food & beverage sector, particularly dairy, beverages, and bottled water, increasingly adopts filter integrity tests to maintain microbial safety. As consumers demand transparency and clean-label assurance, companies are deploying advanced filtration validation systems to meet quality requirements and ensure compliance with stringent global food safety standards.
Technological Advancements Boost Efficiency
Automation, digital monitoring, improved sensors, and data-driven integrity testing solutions are transforming the performance of filter validation. Modern systems offer reduced test times, enhanced accuracy, real-time data logging, and connectivity with manufacturing execution systems (MES), driving higher adoption rates across industries.
Browse Full Report: https://www.factmr.com/report/filter-integrity-test-market
Regional Growth Highlights
North America: Market Leader with Strong Regulatory Framework
The U.S. and Canada exhibit high adoption rates due to a mature pharmaceutical and biotechnology ecosystem. Robust FDA oversight, strong R&D investments, and significant presence of bioprocessing companies drive continuous demand for filter integrity testing equipment.
Europe: Robust Growth Driven by Pharma Excellence
Europe’s well-established biologics and sterile manufacturing sectors fuel rapid uptake of filter validation systems. Supportive regulatory structures, sustainable manufacturing initiatives, and expansion of advanced drug production facilities contribute to market acceleration.
Asia-Pacific: Fastest-Growing Regional Market
Countries like China, India, Japan, and South Korea are heavily investing in biomanufacturing capacities. Growing vaccine production, generics manufacturing, and rising food safety awareness position APAC as a major growth frontier for filter integrity test solutions.
Latin America & Middle East: Emerging Adoption
Increasing pharmaceutical production, expanding beverage markets, and rising healthcare investments create new opportunities for filter integrity testing in emerging economies.
Market Segmentation Insights
By Test Type
- Bubble Point Test – Widely adopted for its precision in detecting pore-size characteristics.
- Pressure Decay Test – Preferred for automated, non-destructive filter validation.
- Diffusion Flow Test – Increasingly used for hydrophilic membrane filters.
By End Use
- Pharmaceutical & Biotechnology – Dominant segment due to sterile manufacturing needs.
- Food & Beverage – Growing adoption for microbial safety and product purity.
- Water Treatment – Increasing integration in potable and ultrapure water systems.
- Research Laboratories – Used for quality validation across R&D settings.
Market Challenges
Despite strong growth prospects, several challenges affect the pace of adoption:
- High Cost of Advanced Testing Systems: Price sensitivity persists across small manufacturers and emerging markets.
- Operational Complexity: Skilled technicians are required to handle specialized testing procedures.
- Lack of Standardization: Variability in test methodologies across regions affects global uniformity.
- Maintenance and Calibration Requirements: Ensuring test accuracy demands routine servicing and compliance checks.
Competitive Landscape
The filter integrity test market is moderately competitive, with companies focusing on automation, regulatory compliance features, and enhanced data documentation. Manufacturers are building strategic partnerships with biopharma companies and investing in digital technologies to strengthen product portfolios.
Prominent companies operating in the market include:
- Sartorius AG
- Merck Millipore
- Parker Hannifin Corporation
- Thermo Fisher Scientific
- Donaldson Company, Inc.
- Pall Corporation
- Meissner Filtration Products
These players emphasize equipment reliability, test accuracy, and connectivity to meet evolving industry standards.
Future Outlook: Advancing Toward Intelligent, Automated, and Compliance-Driven Testing
The next decade will see the filter integrity test market evolve toward heightened automation, digital validation, and real-time compliance monitoring. As sterile manufacturing becomes more sophisticated and regulatory oversight tightens, demand for intelligent testing solutions will rise.
Companies that invest in smart testing systems, MES-integrated platforms, and globally standardized testing protocols will gain a strong competitive advantage. The market’s transition toward data-driven filtration validation will play a critical role in improving process reliability across pharmaceuticals, biotechnology, food safety, and industrial applications.