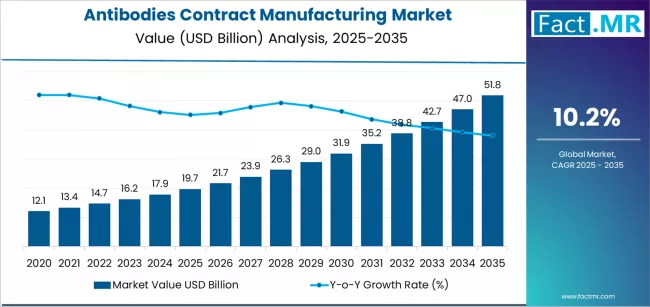
The global antibodies contract manufacturing market is poised for robust expansion over the next decade, fueled by rising biologics development, pharmaceutical outsourcing trends, and rapid advancements in antibody engineering technologies. According to a new analysis by Fact.MR, the market is projected to climb from USD 19.7 billion in 2025 to nearly USD 51.8 billion by 2035, representing an absolute growth of USD 32.1 billion and an impressive CAGR of 10.2% during the forecast period.
With biopharmaceutical companies accelerating R&D in monoclonal antibodies, bispecifics, antibody–drug conjugates (ADCs), and recombinant proteins, outsourcing to specialized contract development and manufacturing organizations (CDMOs) has become a strategic imperative. This shift is driven by the need for cost efficiency, process scalability, regulatory expertise, and access to cutting-edge manufacturing platforms.
Browse Full Report: https://www.factmr.com/report/antibodies-contract-manufacturing-market
Strategic Market Drivers
Biopharma Outsourcing Rises as Antibody Pipelines Expand
The growing therapeutic demand for monoclonal antibodies and next-generation biologics—covering oncology, autoimmune diseases, infectious diseases, cardiovascular disorders, and rare conditions—has increased pressure on pharmaceutical companies to expand antibody production capabilities. CDMOs are stepping in with flexible capacity, high-yield cell culture systems, and turnkey solutions spanning cell-line development to large-scale GMP manufacturing.
Next-Generation Antibody Engineering Accelerates Manufacturing Complexity
The emergence of bispecific antibodies, Fc-engineered antibodies, and antibody fragments has heightened demand for advanced bio-manufacturing platforms. CDMOs are integrating single-use bioreactors, perfusion systems, AI-enabled process analytics, and high-throughput screening technologies to support efficient, scalable, and compliant production of complex biologics.
Global Manufacturing Infrastructure Strengthens Market Penetration
North America: Innovation and Biopharma Leadership
North America remains the powerhouse market owing to strong biologics pipelines, availability of large CDMOs, and rapid adoption of high-intensity bioprocessing technologies. The U.S. leads in clinical antibody development, driving sustained manufacturing demand.
Europe: Strong Regulatory Framework and High CDMO Capabilities
Germany, Switzerland, the U.K., and France host world-class biologics manufacturing facilities. Europe benefits from a well-defined regulatory regime, advanced bioprocess technologies, and growing investments in flexible manufacturing capacity.
East Asia: Fast-Expanding Biomanufacturing Ecosystem
China, Japan, and South Korea are emerging as global hubs for biologics manufacturing, driven by government support, strong capacity expansions, and significant biopharma investments. Regional CDMOs are scaling up to serve both domestic and global pipelines.
Emerging Markets: High Growth Potential
India, Singapore, Brazil, and the Middle East are witnessing growing demand for antibody therapeutics and expanding local manufacturing footprints. Increasing clinical trial activity and foreign partnerships position these regions for strong future growth.
Market Segmentation Insights
By Type of Antibody
- Monoclonal Antibodies (mAbs) – Dominant segment due to extensive therapeutic use
- Polyclonal Antibodies – Niche but essential for research, diagnostics
- Antibody Fragments & Biospecifics – Fastest-growing segment driven by targeted therapies
- Antibody–Drug Conjugates (ADCs) – High-value, high-complexity manufacturing
By Service Type
- Cell Line Development
- Process Development & Optimization
- Upstream & Downstream GMP Manufacturing
- Fill-Finish Services
- Analytical & Regulatory Support
By Expression System
- Mammalian Expression (CHO cells dominate)
- Microbial Expression
- Plant-Based & Cell-Free Systems (emerging)
By End User
- Biopharma Companies
- Biotechnology Firms
- Research Institutes
- CROs & Startups
Key Challenges Affecting Market Growth
- High Manufacturing Costs – Complex bioprocessing increases CAPEX and OPEX
- Quality & Regulatory Compliance Pressures – Strict FDA and EMA standards
- Capacity Constraints in Peak Demand Periods
- Competition from In-House Manufacturing – Large pharma companies expanding internal capacity
- Supply Chain Vulnerabilities – Dependence on critical materials, consumables, and global logistics
Competitive Landscape
The antibodies contract manufacturing market is moderately concentrated, with global CDMOs investing heavily in capacity expansion, single-use technologies, and end-to-end biologics development platforms.
Key Companies Profiled:
• Lonza Group
• Samsung Biologics
• Boehringer Ingelheim BioXcellence
• WuXi Biologics
• Catalent
• Thermo Fisher Scientific
• Fujifilm Diosynth Biotechnologies
• AbbVie Contract Manufacturing
• Novasep
• Rentschler Biopharma
These companies focus on high-throughput platforms, high-density cell culture, integrated development-to-commercialization pipelines, and global manufacturing footprints.
Recent Industry Developments
- 2024: CDMOs expanded capacity for ADCs, bispecific antibodies, and continuous bioprocessing.
- 2023: Multiple players introduced AI-driven bioprocess optimization tools for accelerated scale-up.
- 2022: Global investments surged in single-use bioreactors and high-capacity facilities across the U.S., Europe, China, and South Korea.
Future Outlook: A Decade of Biologics Manufacturing Transformation
The antibodies contract manufacturing market is set for transformative growth as biologics continue to dominate drug pipelines and next-generation antibody formats gain approvals. CDMOs that invest in digital bioprocessing, flexible modular facilities, sustainability, and integrated service portfolios will capture significant market share.
With expanding therapeutic demand, increasing outsourcing strategies, and rapid technological advancements, the market is well positioned for strong, sustained growth through 2035.