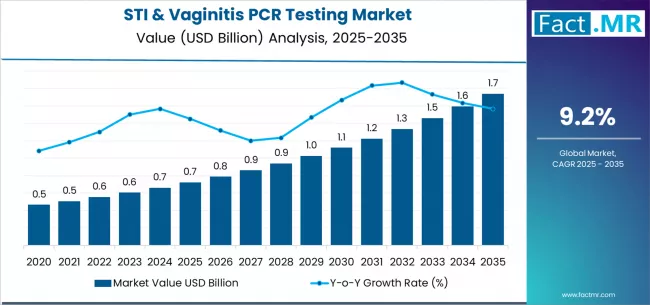
The global STI & vaginitis PCR testing market is witnessing significant growth as healthcare systems increasingly prioritize accurate and rapid diagnostics for sexual and reproductive health. Polymerase Chain Reaction (PCR)–based testing enables precise pathogen detection for sexually transmitted infections (STIs) and vaginal infections, offering high sensitivity and specificity compared to traditional diagnostic methods. The rising prevalence of STIs, increasing awareness about sexual health, and adoption of advanced molecular diagnostic tools are key factors driving the demand for PCR testing across clinical laboratories, hospitals, and point-of-care facilities.
Market Overview
STI & vaginitis PCR testing encompasses multiplex panels and specialized assays that identify bacterial, viral, and parasitic pathogens responsible for infections. Multiplex STI PCR panels, which allow simultaneous detection of multiple pathogens such as chlamydia, gonorrhea, and trichomoniasis, dominate the market. Vaginitis PCR panels target common vaginal infections, including bacterial vaginosis and yeast infections, providing rapid results to guide effective treatment decisions. The versatility and high accuracy of PCR testing make it a preferred choice for routine screening, symptomatic evaluation, and preventive care programs.
The growing focus on antimicrobial stewardship has further reinforced the need for PCR diagnostics. By ensuring targeted therapy based on precise pathogen identification, PCR testing reduces unnecessary antibiotic usage and helps mitigate the rise of antimicrobial resistance. Moreover, the integration of PCR testing with electronic health records and laboratory information systems enhances workflow efficiency and clinical decision-making.
Regional Insights
North America leads the STI & vaginitis PCR testing market, driven by well-established healthcare infrastructure, robust laboratory networks, and high awareness of sexual health. Europe follows closely, supported by government initiatives and widespread adoption of molecular diagnostics. The Asia Pacific region is emerging as a strong growth market due to expanding healthcare access, increasing public health investments, and rising prevalence of STIs. Other regions, including Latin America and the Middle East & Africa, are gradually increasing adoption, driven by rising awareness and infrastructure development.
Key Trends & Drivers
Several trends are shaping the STI & vaginitis PCR testing market:
- Rise of Multiplex Testing:Multiplex PCR panels enable simultaneous detection of multiple pathogens, enhancing diagnostic efficiency and reducing turnaround times.
- Integration with Digital Health Systems:Combining PCR testing with electronic health records improves data management, patient tracking, and treatment accuracy.
- Focus on Women’s Health:The increasing emphasis on preventive screening and reproductive health drives demand for specialized vaginal infection PCR panels.
- Point-of-Care Expansion:Rapid PCR platforms are gaining traction in clinics, community healthcare centers, and home-based testing setups.
- Antimicrobial Resistance Monitoring:PCR testing supports targeted therapy selection, helping healthcare providers combat the rise of resistant infections.
Market Dynamics
The STI & vaginitis PCR testing market is fueled by rising investments in sexual health infrastructure and growing demand for precise diagnostics. Clinical laboratories and hospitals are increasingly adopting automated molecular platforms to expand testing capacity, streamline workflows, and ensure consistent results. Preventive health programs and screening initiatives also contribute to market growth by encouraging routine testing in at-risk populations.
Despite strong growth, challenges remain. High costs associated with comprehensive PCR panels, reimbursement constraints, and technical expertise requirements may limit adoption in smaller healthcare facilities and resource-limited regions. Ensuring sample stability, proper collection protocols, and accurate result interpretation are critical factors for successful implementation.
Opportunities and Innovations
The market offers opportunities in next-generation PCR platforms, including rapid nucleic acid amplification and metagenomic pathogen detection. Innovations in home-based testing, decentralized laboratories, and point-of-care diagnostics are expanding access to high-quality STI and vaginitis testing. Additionally, the development of integrated multiplex panels that combine STI and vaginitis testing with antimicrobial resistance profiling provides a holistic approach to sexual health management.
Competitive Landscape
Leading companies in the STI & vaginitis PCR testing market include BD, Roche Diagnostics, Hologic, Abbott, Cepheid (Danaher), Seegene, bioMérieux (BioFire), QIAGEN, Thermo Fisher Scientific, and DiaSorin (Luminex). These players focus on product innovation, regulatory approvals, and expanding distribution networks to strengthen their market presence. Strategic partnerships with healthcare providers and investments in research and development further support the adoption of advanced PCR testing solutions.
Applications & End-Use Outlook
PCR testing for STIs and vaginitis is widely applied in diagnostic laboratories, hospitals, and clinics, with emerging use in home-based testing solutions. Laboratories leverage multiplex panels for routine screening and symptomatic evaluation, while hospitals integrate PCR diagnostics into infectious disease management protocols. Home-based and point-of-care testing expands access to underserved populations, ensuring timely diagnosis and treatment.
Conclusion
The STI & vaginitis PCR testing market is poised for sustained growth, driven by rising awareness of sexual health, adoption of molecular diagnostic technologies, and increasing public health investments. Multiplex PCR panels, rapid testing platforms, and next-generation diagnostic innovations are reshaping clinical workflows and improving patient outcomes. Despite challenges related to cost and technical expertise, the market presents significant opportunities for expanding access to high-quality, precise, and timely diagnostic solutions in sexual and reproductive healthcare. Stakeholders investing in innovative PCR technologies, streamlined workflows, and preventive health initiatives are well-positioned to capitalize on this growing market.
Browse Full Report – https://www.factmr.com/report/sti-vaginitis-pcr-testing-market