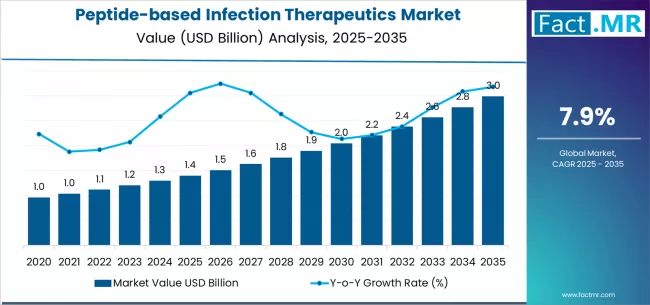
The global peptide-based infection therapeutics market stands at the threshold of a decade-long expansion trajectory that promises to reshape antimicrobial treatment and infection management solutions. According to a recent report by Fact.MR, the market is projected to grow from USD 1.4 billion in 2025 to USD 3.0 billion by 2035, reflecting a CAGR of 7.9% during the forecast period.
Peptide-based infection therapeutics are emerging as a breakthrough class of biologically inspired drugs designed to overcome the limitations of conventional antibiotics. Their high specificity, low toxicity, and reduced resistance potential position them as next-generation solutions for combating multidrug-resistant pathogens across hospital and community settings.
Strategic Market Drivers
Rising Antimicrobial Resistance (AMR): A Global Health Crisis
The surge in antimicrobial resistance has catalyzed a wave of innovation in therapeutic peptides. With bacteria increasingly evading traditional antibiotics, peptide-based drugs—offering mechanisms such as membrane disruption and immune modulation—are becoming the cornerstone of next-generation anti-infective therapies. Governments and global health agencies are accelerating funding to expand R&D pipelines focused on novel peptide compounds.
Expanding Research and Biotech Collaborations
Collaborative efforts between pharmaceutical giants, biotech startups, and academic institutions are accelerating the development of novel peptide molecules. Advances in peptide synthesis, stability enhancement, and delivery technologies are improving drug efficacy and scalability. Licensing agreements and joint ventures are driving rapid clinical progress, with multiple candidates advancing through late-stage trials.
Growing Applications in Nosocomial and Chronic Infections
Peptide-based therapeutics are increasingly used to address high-burden infections such as methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and Clostridioides difficile. Beyond acute infections, research is expanding into chronic wound management and biofilm-associated diseases, broadening clinical and commercial opportunities.
Regulatory Incentives and Fast-Track Approvals
Global regulatory bodies are promoting fast-track and orphan drug designations to encourage peptide-based infection therapy development. Initiatives from the U.S. FDA, EMA, and WHO are fostering accelerated clinical pathways, supporting innovation while addressing unmet medical needs in resistant bacterial and fungal infections.
Browse Full Report: https://www.factmr.com/report/188/peptide-based-infection-therapeutics-market
Regional Growth Highlights
North America: Leading with Innovation and Clinical Trials
The U.S. leads the global market, driven by high AMR prevalence, strong government funding, and advanced clinical research infrastructure. Strategic partnerships between biotech innovators and major pharmaceutical firms are boosting late-stage pipeline activity. Canada’s growing focus on precision medicine and biologics is also reinforcing regional growth.
Europe: Regulation-Driven Research Acceleration
Europe continues to drive policy-led innovation, with the European Medicines Agency (EMA) supporting streamlined approval for antimicrobial peptides under the “New Antimicrobial Action Plan.” The U.K., Germany, and Switzerland remain pivotal hubs for peptide synthesis and formulation research.
Asia Pacific: Emerging as a High-Growth Hub
Rapid healthcare infrastructure development and government investments in biotechnology are propelling Asia Pacific’s market expansion. China, Japan, and South Korea are increasingly investing in localized peptide drug production and AMR-focused research collaborations.
Latin America & Middle East: Untapped Potential
Rising infection rates, expanding healthcare access, and growing awareness about advanced therapeutics are setting the stage for emerging market expansion. Brazil, Saudi Arabia, and the UAE are expected to become focal points for future market penetration and public-private healthcare partnerships.
Market Segmentation Insights
By Drug Type
- Antimicrobial Peptides (AMPs) – Driving the market as frontline treatments for resistant bacterial infections.
- Immunomodulatory Peptides – Gaining traction for dual-action roles in infection control and inflammation reduction.
- Synthetic and Hybrid Peptides – Offering improved bioavailability and resistance resilience through molecular engineering.
By Infection Type
- Bacterial Infections – Largest segment due to growing multidrug resistance.
- Viral and Fungal Infections – Emerging applications in opportunistic and chronic infections.
- Nosocomial and Community-Acquired Infections – Increasing hospital adoption for high-risk patient populations.
By End User
- Hospitals and Clinics – Primary consumers for critical infection management.
- Research Institutes and Biopharma Companies – Major contributors to ongoing drug discovery and trials.
Challenges and Market Considerations
Despite strong growth potential, the market faces key challenges:
- High Development Costs: Complex peptide synthesis and stability optimization increase R&D expenses.
- Regulatory and Reimbursement Barriers: Long approval timelines and limited reimbursement pathways slow commercialization.
- Manufacturing Scalability: Achieving large-scale, cost-efficient peptide production remains a critical bottleneck.
Competitive Landscape
The peptide-based infection therapeutics market is innovation-driven, characterized by strong R&D investments, clinical partnerships, and advanced formulation strategies.
Key Players in the Peptide-based Infection Therapeutics Market:
- Melinta Therapeutics, Inc. / IQVIA Peptide Assets
- Pfizer Inc.
- Merck & Co., Inc.
- Roche Holding AG
- Polyphor Ltd. (Legacy Assets)
- Spero Therapeutics, Inc.
- Destiny Pharma plc
- Summit Therapeutics plc
- ContraFect Corporation
- Basilea Pharmaceutica International Ltd.
These companies are focusing on clinical-stage advancements, licensing deals, and next-generation antimicrobial discovery to expand market presence.
Recent Developments
- June 2023 – Melinta Therapeutics advanced its peptide-based antibacterial candidate targeting resistant Gram-negative bacteria into Phase II clinical trials.
- March 2023 – Destiny Pharma plc announced strategic collaboration to develop cationic antimicrobial peptides (CAMPs) for chronic wound infections.
- January 2024 – Pfizer expanded its infectious disease R&D portfolio by integrating peptide-based therapeutics through strategic acquisitions.
Future Outlook: Redefining the Fight Against Infection
The next decade will mark a transformative era for peptide-based infection therapeutics. As the global healthcare system shifts toward biologically inspired and precision-based treatments, peptides are poised to become central to modern infection control. Advances in AI-driven peptide design, sustainable biomanufacturing, and nanotechnology-based delivery systems will accelerate clinical success and market adoption.
With growing urgency to combat antimicrobial resistance and deliver safer, more effective treatments, the peptide-based infection therapeutics market is set to redefine the global infection management paradigm—driving healthcare toward a smarter, safer, and more resilient future.