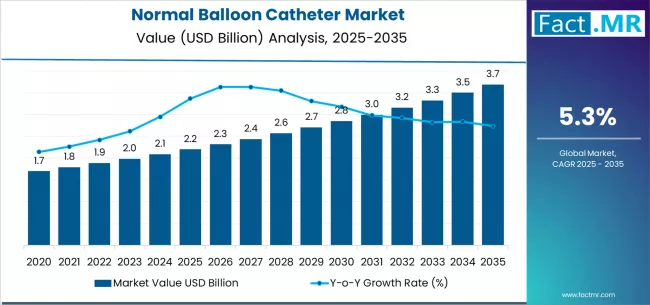
The global normal balloon catheter market is poised for robust expansion over the next decade, underpinned by increasing cardiovascular disease prevalence and accelerating adoption of minimally invasive procedures.
According to a recent report by Fact.MR, the market is projected to grow from USD 2.2 billion in 2025 to approximately USD 3.7 billion by 2035, recording an absolute increase of USD 1.5 billion over the forecast period. This translates to a total growth of 68.2%, with a CAGR of 5.3% between 2025 and 2035. Overall, the market size is expected to grow by nearly 1.7 times during the same period.
Key Market Drivers
Rising Burden of Cardiovascular Diseases
The growing incidence of coronary artery disease, peripheral vascular disorders, and related complications continues to be the primary demand driver for normal balloon catheters. Globally, cardiovascular diseases remain the leading cause of mortality, driving hospitals and clinics to adopt advanced catheterization technologies that offer precision, safety, and cost efficiency.
Shift Toward Minimally Invasive Interventions
Normal balloon catheters play a critical role in angioplasty and stent placement procedures that prioritize patient recovery and reduced hospital stays. The increasing preference for minimally invasive cardiovascular procedures has substantially fueled the adoption of high-quality balloon catheters, particularly in aging populations with comorbidities.
Technological Advancements and Product Innovation
Manufacturers are introducing next-generation balloon catheters featuring improved flexibility, smaller profiles, enhanced trackability, and superior balloon compliance. These innovations are enabling physicians to navigate complex vascular anatomies with greater precision and safety, ultimately improving procedural outcomes.
Growing Adoption in Emerging Healthcare Systems
Rapid healthcare infrastructure expansion in Asia-Pacific, Latin America, and the Middle East is creating new avenues for market growth. Public health initiatives promoting early diagnosis and treatment of cardiovascular conditions are encouraging broader catheter utilization in both public and private hospitals.
Browse Full Report: https://www.factmr.com/report/147/normal-balloon-catheter-market
Regional Growth Highlights
North America: Advanced Infrastructure and R&D Leadership
North America remains a dominant market, driven by high procedural volumes, technological innovation, and the strong presence of major device manufacturers. The U.S. continues to witness growth in cardiovascular interventions, supported by favorable reimbursement frameworks and continuous product innovation in interventional cardiology devices.
Europe: Regulatory Strength and Product Safety Standards
Europe’s market growth is influenced by stringent safety regulations and an emphasis on clinical efficacy. Countries such as Germany, the U.K., and France are key hubs for innovation, focusing on improved biocompatibility and patient-specific catheter designs to enhance procedural precision and reduce restenosis risks.
Asia-Pacific: Fastest-Growing Market
Asia-Pacific is emerging as the most rapidly expanding region, supported by rising healthcare spending, growing medical tourism, and increasing awareness of cardiovascular health. Nations like China, India, and Japan are driving adoption due to high patient volumes and expanding catheterization lab networks.
Latin America & Middle East: Emerging Potential
Increased government healthcare funding, infrastructure modernization, and rising cardiac screening programs are fostering market expansion across Brazil, Mexico, and GCC countries, signaling strong long-term opportunities for catheter manufacturers.
Market Segmentation Insights
By Application
- Coronary Angioplasty – Largest segment, driven by the high prevalence of coronary artery disease.
- Peripheral Angioplasty – Witnessing growth due to the rising burden of peripheral arterial disease (PAD).
- Other Applications – Including dialysis access and neurovascular procedures.
By End User
- Hospitals – Major end users owing to advanced cardiac units and higher procedural throughput.
- Cardiac Catheterization Labs – Increasing adoption of specialized devices for high-precision interventions.
- Ambulatory Surgical Centers (ASCs) – Emerging as cost-effective alternatives for low-risk procedures.
Key Challenges
- Pricing Pressure and Reimbursement Constraints: Cost sensitivity in developing regions and complex reimbursement pathways limit broader accessibility.
- Procedural Risks and Regulatory Compliance: Stringent FDA and CE mark requirements necessitate ongoing R&D investments.
- Competition from Drug-Coated and Specialty Catheters: Advanced alternatives may limit the growth of standard balloon catheter segments in mature markets.
Key Players in the Normal Balloon Catheter Market
- Boston Scientific Corporation
- Medtronic plc
- Abbott Laboratories
- Terumo Corporation
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Cordis (Cardinal Health)
- Cook Medical
- Teleflex Incorporated
- Biotronik SE & Co. KG
These leading companies are expanding their global footprints through strategic collaborations, mergers, and technological partnerships. Focus areas include smaller balloon profiles, enhanced crossability, and hybrid materials that improve catheter performance and patient safety.
Future Outlook: Advancing Cardiovascular Efficiency Through Smart Catheter Design
The next decade will mark a pivotal transformation for the normal balloon catheter market, characterized by enhanced product intelligence, sustainable materials, and precision-driven performance. Integration of AI-assisted navigation, real-time imaging compatibility, and digital performance analytics will redefine procedural efficiency and safety.
As the world faces a rising cardiovascular burden, manufacturers that invest in innovation, affordability, and regional accessibility will shape the next wave of growth—enabling improved patient outcomes and driving the evolution of interventional cardiology across global healthcare systems.